Business Potential for Agricultural Biotechnology - Asian Productivity ...
Business Potential for Agricultural Biotechnology - Asian Productivity ...
Business Potential for Agricultural Biotechnology - Asian Productivity ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Business</strong> <strong>Potential</strong> <strong>for</strong> <strong>Agricultural</strong> <strong>Biotechnology</strong> Products<br />
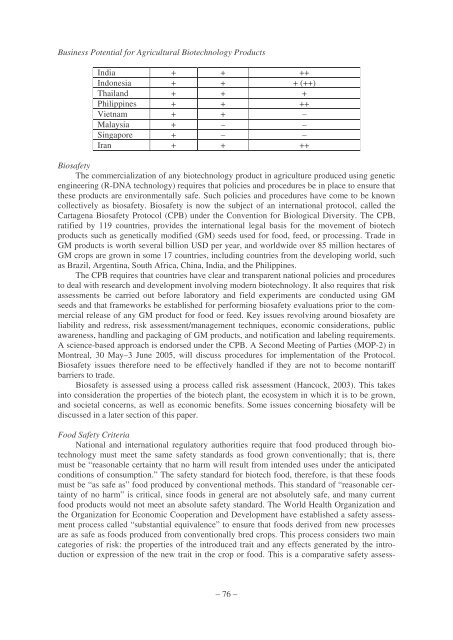
India + + ++<br />
Indonesia + + + (++)<br />
Thailand + + +<br />
Philippines + + ++<br />
Vietnam + + –<br />
Malaysia + – –<br />
Singapore + – –<br />
Iran + + ++<br />
Biosafety<br />
The commercialization of any biotechnology product in agriculture produced using genetic<br />
engineering (R-DNA technology) requires that policies and procedures be in place to ensure that<br />
these products are environmentally safe. Such policies and procedures have come to be known<br />
collectively as biosafety. Biosafety is now the subject of an international protocol, called the<br />
Cartagena Biosafety Protocol (CPB) under the Convention <strong>for</strong> Biological Diversity. The CPB,<br />
ratified by 119 countries, provides the international legal basis <strong>for</strong> the movement of biotech<br />
products such as genetically modified (GM) seeds used <strong>for</strong> food, feed, or processing. Trade in<br />
GM products is worth several billion USD per year, and worldwide over 85 million hectares of<br />
GM crops are grown in some 17 countries, including countries from the developing world, such<br />
as Brazil, Argentina, South Africa, China, India, and the Philippines.<br />
The CPB requires that countries have clear and transparent national policies and procedures<br />
to deal with research and development involving modern biotechnology. It also requires that risk<br />
assessments be carried out be<strong>for</strong>e laboratory and field experiments are conducted using GM<br />
seeds and that frameworks be established <strong>for</strong> per<strong>for</strong>ming biosafety evaluations prior to the commercial<br />
release of any GM product <strong>for</strong> food or feed. Key issues revolving around biosafety are<br />
liability and redress, risk assessment/management techniques, economic considerations, public<br />
awareness, handling and packaging of GM products, and notification and labeling requirements.<br />
A science-based approach is endorsed under the CPB. A Second Meeting of Parties (MOP-2) in<br />
Montreal, 30 May–3 June 2005, will discuss procedures <strong>for</strong> implementation of the Protocol.<br />
Biosafety issues there<strong>for</strong>e need to be effectively handled if they are not to become nontariff<br />
barriers to trade.<br />
Biosafety is assessed using a process called risk assessment (Hancock, 2003). This takes<br />
into consideration the properties of the biotech plant, the ecosystem in which it is to be grown,<br />
and societal concerns, as well as economic benefits. Some issues concerning biosafety will be<br />
discussed in a later section of this paper.<br />
Food Safety Criteria<br />
National and international regulatory authorities require that food produced through biotechnology<br />
must meet the same safety standards as food grown conventionally; that is, there<br />
must be “reasonable certainty that no harm will result from intended uses under the anticipated<br />
conditions of consumption.” The safety standard <strong>for</strong> biotech food, there<strong>for</strong>e, is that these foods<br />
must be “as safe as” food produced by conventional methods. This standard of “reasonable certainty<br />
of no harm” is critical, since foods in general are not absolutely safe, and many current<br />
food products would not meet an absolute safety standard. The World Health Organization and<br />
the Organization <strong>for</strong> Economic Cooperation and Development have established a safety assessment<br />
process called “substantial equivalence” to ensure that foods derived from new processes<br />
are as safe as foods produced from conventionally bred crops. This process considers two main<br />
categories of risk: the properties of the introduced trait and any effects generated by the introduction<br />
or expression of the new trait in the crop or food. This is a comparative safety assess-<br />
– 76 –