Tutorials Manual
Tutorials Manual
Tutorials Manual
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chemkin 4.1.1<br />
Chapter 2: Combustion in Gas-phase Processes<br />
In Figure 2-9, there is an obvious difference (about a factor of four) between the<br />
ignition time values as defined by the two chosen criteria. This plot demonstrates why<br />
one should be mindful of ignition time definitions used in computations. The dark line<br />
is the temperature profile and the light line is the OH mole fraction.<br />
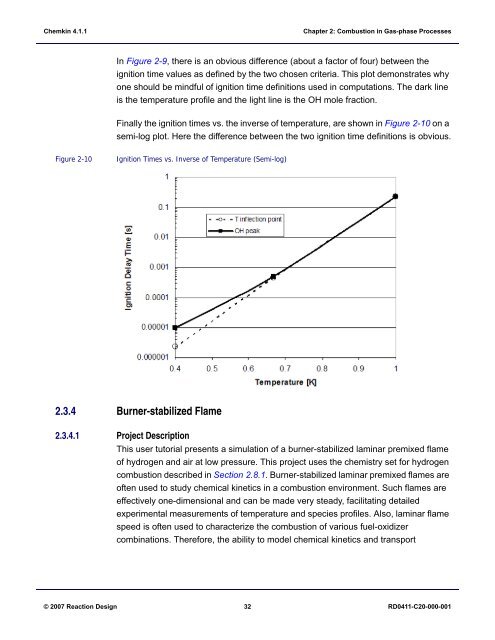
Finally the ignition times vs. the inverse of temperature, are shown in Figure 2-10 on a<br />
semi-log plot. Here the difference between the two ignition time definitions is obvious.<br />
Figure 2-10<br />
Ignition Times vs. Inverse of Temperature (Semi-log)<br />
2.3.4 Burner-stabilized Flame<br />
2.3.4.1 Project Description<br />
This user tutorial presents a simulation of a burner-stabilized laminar premixed flame<br />
of hydrogen and air at low pressure. This project uses the chemistry set for hydrogen<br />
combustion described in Section 2.8.1. Burner-stabilized laminar premixed flames are<br />
often used to study chemical kinetics in a combustion environment. Such flames are<br />
effectively one-dimensional and can be made very steady, facilitating detailed<br />
experimental measurements of temperature and species profiles. Also, laminar flame<br />
speed is often used to characterize the combustion of various fuel-oxidizer<br />
combinations. Therefore, the ability to model chemical kinetics and transport<br />
© 2007 Reaction Design 32 RD0411-C20-000-001