Tutorials Manual
Tutorials Manual
Tutorials Manual
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 2: Combustion in Gas-phase Processes<br />
<strong>Tutorials</strong> <strong>Manual</strong><br />
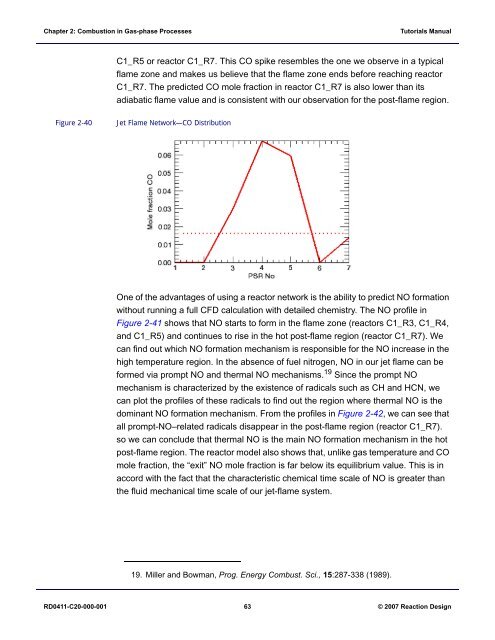
C1_R5 or reactor C1_R7. This CO spike resembles the one we observe in a typical<br />
flame zone and makes us believe that the flame zone ends before reaching reactor<br />
C1_R7. The predicted CO mole fraction in reactor C1_R7 is also lower than its<br />
adiabatic flame value and is consistent with our observation for the post-flame region.<br />
Figure 2-40<br />
Jet Flame Network—CO Distribution<br />
One of the advantages of using a reactor network is the ability to predict NO formation<br />
without running a full CFD calculation with detailed chemistry. The NO profile in<br />
Figure 2-41 shows that NO starts to form in the flame zone (reactors C1_R3, C1_R4,<br />
and C1_R5) and continues to rise in the hot post-flame region (reactor C1_R7). We<br />
can find out which NO formation mechanism is responsible for the NO increase in the<br />
high temperature region. In the absence of fuel nitrogen, NO in our jet flame can be<br />
formed via prompt NO and thermal NO mechanisms. 19 Since the prompt NO<br />
mechanism is characterized by the existence of radicals such as CH and HCN, we<br />
can plot the profiles of these radicals to find out the region where thermal NO is the<br />
dominant NO formation mechanism. From the profiles in Figure 2-42, we can see that<br />
all prompt-NO–related radicals disappear in the post-flame region (reactor C1_R7).<br />
so we can conclude that thermal NO is the main NO formation mechanism in the hot<br />
post-flame region. The reactor model also shows that, unlike gas temperature and CO<br />
mole fraction, the “exit” NO mole fraction is far below its equilibrium value. This is in<br />
accord with the fact that the characteristic chemical time scale of NO is greater than<br />
the fluid mechanical time scale of our jet-flame system.<br />
19. Miller and Bowman, Prog. Energy Combust. Sci., 15:287-338 (1989).<br />
RD0411-C20-000-001 63 © 2007 Reaction Design