- Page 2 and 3:
BIOTECHNOLOGY IN AGRICULTURE, INDUS
- Page 4:
Industrial Biotechnology Shara L. A
- Page 7 and 8:
Copyright © 2009 by Nova Science P
- Page 9 and 10:
viii Contents Chapter 8 Pharmacolog
- Page 11 and 12:
x Alejandro Varela and Jasiah Ibañ
- Page 13 and 14:
xii Alejandro Varela and Jasiah Iba
- Page 15 and 16:
xiv Alejandro Varela and Jasiah Iba
- Page 18 and 19:
In: Medicinal Plants: Classificatio
- Page 20 and 21:
Biological Effects of -Carotene acy
- Page 22 and 23:
Biological Effects of -Carotene cry
- Page 24 and 25:
Biological Effects of -Carotene ris
- Page 26 and 27:
Biological Effects of -Carotene Whe
- Page 28 and 29:
Biological Effects of -Carotene fla
- Page 30 and 31:
Biological Effects of -Carotene Pre
- Page 32 and 33:
Biological Effects of -Carotene A (
- Page 34 and 35:
Biological Effects of -Carotene As
- Page 36 and 37:
Biological Effects of -Carotene car
- Page 38 and 39:
Biological Effects of -Carotene res
- Page 40 and 41:
Biological Effects of -Carotene the
- Page 42 and 43:
Biological Effects of -Carotene cat
- Page 44 and 45:
Biological Effects of -Carotene mos
- Page 46 and 47:
Biological Effects of -Carotene dia
- Page 48 and 49:
Biological Effects of -Carotene veg
- Page 50 and 51:
Biological Effects of -Carotene .Su
- Page 52 and 53:
Biological Effects of -Carotene Bat
- Page 54 and 55:
Biological Effects of -Carotene Dah
- Page 56 and 57:
Biological Effects of -Carotene Hal
- Page 58 and 59:
Biological Effects of -Carotene Kva
- Page 60 and 61:
Biological Effects of -Carotene Osh
- Page 62 and 63:
Biological Effects of -Carotene San
- Page 64:
Biological Effects of -Carotene Van
- Page 67 and 68:
50 Gustavo J. Martínez, Mara Sato
- Page 69 and 70:
52 Gustavo J. Martínez, Mara Sato
- Page 71 and 72:
54 Gustavo J. Martínez, Mara Sato
- Page 73 and 74:
56 Gustavo J. Martínez, Mara Sato
- Page 75 and 76:
58 [2] Gustavo J. Martínez, Mara S
- Page 77 and 78:
60 Gustavo J. Martínez, Mara Sato
- Page 79 and 80:
62 Gustavo J. Martínez, Mara Sato
- Page 81 and 82:
64 Gustavo J. Martínez, Mara Sato
- Page 83 and 84:
66 Gustavo J. Martínez, Mara Sato
- Page 85 and 86:
68 Gustavo J. Martínez, Mara Sato
- Page 87 and 88:
70 Gustavo J. Martínez, Mara Sato
- Page 89 and 90:
Huperzia saururus (Lam.) Trevis. Mi
- Page 91 and 92:
74 Gustavo J. Martínez, Mara Sato
- Page 93 and 94:
76 Gustavo J. Martínez, Mara Sato
- Page 95 and 96:
PNEUMONOLOGY AND INFECTIOUS DISEASE
- Page 97 and 98:
80 Gustavo J. Martínez, Mara Sato
- Page 99 and 100:
82 Gustavo J. Martínez, Mara Sato
- Page 101 and 102:
84 Gustavo J. Martínez, Mara Sato
- Page 103 and 104:
86 Gustavo J. Martínez, Mara Sato
- Page 105 and 106:
88 Gustavo J. Martínez, Mara Sato
- Page 107 and 108:
90 Gustavo J. Martínez, Mara Sato
- Page 109 and 110:
92 Gustavo J. Martínez, Mara Sato
- Page 111 and 112:
94 Gustavo J. Martínez, Mara Sato
- Page 113 and 114:
96 Gustavo J. Martínez, Mara Sato
- Page 115 and 116:
98 Aracely E. Chávez-Piña and And
- Page 117 and 118:
100 Aracely E. Chávez-Piña and An
- Page 119 and 120:
102 Aracely E. Chávez-Piña and An
- Page 121 and 122:
104 Aracely E. Chávez-Piña and An
- Page 123 and 124:
106 Aracely E. Chávez-Piña and An
- Page 125 and 126:
108 Aracely E. Chávez-Piña and An
- Page 127 and 128:
110 Aracely E. Chávez-Piña and An
- Page 129 and 130:
112 Aracely E. Chávez-Piña and An
- Page 131 and 132:
114 Aracely E. Chávez-Piña and An
- Page 133 and 134:
116 Aracely E. Chávez-Piña and An
- Page 135 and 136:
118 HO HO HO HO HO HO OH OH M e Me
- Page 137 and 138:
120 Me Me OH Me O OH O O OH H Me OH
- Page 139 and 140:
122 HO HO HO Aracely E. Chávez-Pi
- Page 141 and 142:
124 Aracely E. Chávez-Piña and An
- Page 143 and 144:
126 Aracely E. Chávez-Piña and An
- Page 145 and 146:
128 Aracely E. Chávez-Piña and An
- Page 147 and 148:
130 Aracely E. Chávez-Piña and An
- Page 149 and 150:
132 Aracely E. Chávez-Piña and An
- Page 151 and 152:
134 Aracely E. Chávez-Piña and An
- Page 153 and 154:
136 Aracely E. Chávez-Piña and An
- Page 155 and 156:
138 Aracely E. Chávez-Piña and An
- Page 157 and 158:
140 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 159 and 160:
142 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 161 and 162:
144 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 163 and 164:
146 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 165 and 166:
148 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 167 and 168:
150 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 169 and 170:
152 6.4. Antibacterial Effects Hua-
- Page 171 and 172:
154 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 173 and 174:
156 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 175 and 176:
158 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 177 and 178:
160 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 179 and 180:
162 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 181 and 182:
164 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 183 and 184:
166 Hua-Bin Li, Dan Li, Ren-You Gan
- Page 185 and 186:
168 Vandana Gulati, Ian H. Harding
- Page 187 and 188:
170 Vandana Gulati, Ian H. Harding
- Page 189 and 190:
172 Vandana Gulati, Ian H. Harding
- Page 191 and 192:
174 Vandana Gulati, Ian H. Harding
- Page 193 and 194:
176 Vandana Gulati, Ian H. Harding
- Page 195 and 196:
178 Vandana Gulati, Ian H. Harding
- Page 197 and 198:
180 Vandana Gulati, Ian H. Harding
- Page 199 and 200: 182 Vandana Gulati, Ian H. Harding
- Page 201 and 202: 184 Vandana Gulati, Ian H. Harding
- Page 203 and 204: 186 Vandana Gulati, Ian H. Harding
- Page 206 and 207: In: Medicinal Plants: Classificatio
- Page 208 and 209: Biomolecules with Anti-Mycobacteria
- Page 210 and 211: Biomolecules with Anti-Mycobacteria
- Page 212 and 213: Biomolecules with Anti-Mycobacteria
- Page 214 and 215: Biomolecules with Anti-Mycobacteria
- Page 216 and 217: Biomolecules with Anti-Mycobacteria
- Page 218: Biomolecules with Anti-Mycobacteria
- Page 221 and 222: 204 Chien-Yi Chen Ligusticum chuanx
- Page 223 and 224: 206 Chien-Yi Chen (IAEA 336), also
- Page 225 and 226: 208 Chien-Yi Chen 4. Results and Di
- Page 227 and 228: 210 4.3. Fresh Leaves and Stems Chi
- Page 229 and 230: 212 Chien-Yi Chen collected from na
- Page 231 and 232: 214 Chien-Yi Chen primary enzyme in
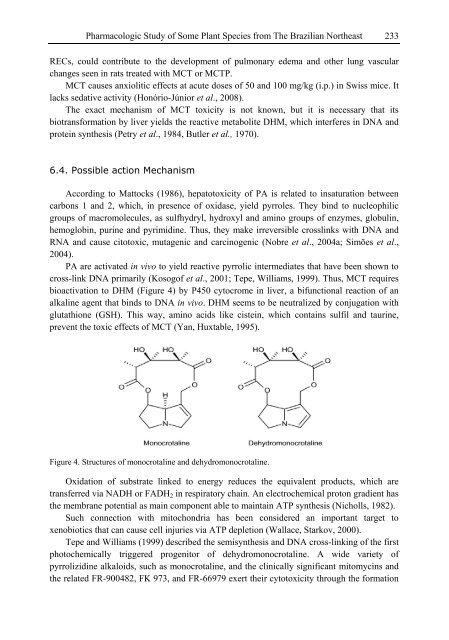
- Page 233 and 234: 216 Chien-Yi Chen [20] Liu SM, Chun
- Page 235 and 236: 218 S.M.M. Vasconcelos, J.E.R. Hon
- Page 237 and 238: 220 S.M.M. Vasconcelos, J.E.R. Hon
- Page 239 and 240: 222 S.M.M. Vasconcelos, J.E.R. Hon
- Page 241 and 242: 224 S.M.M. Vasconcelos, J.E.R. Hon
- Page 243 and 244: 226 S.M.M. Vasconcelos, J.E.R. Hon
- Page 245 and 246: 228 S.M.M. Vasconcelos, J.E.R. Hon
- Page 247 and 248: 230 S.M.M. Vasconcelos, J.E.R. Hon
- Page 249: 232 S.M.M. Vasconcelos, J.E.R. Hon
- Page 253 and 254: 236 S.M.M. Vasconcelos, J.E.R. Hon
- Page 255 and 256: 238 S.M.M. Vasconcelos, J.E.R. Hon
- Page 257 and 258: 240 S.M.M. Vasconcelos, J.E.R. Hon
- Page 259 and 260: 242 S.M.M. Vasconcelos, J.E.R. Hon
- Page 261 and 262: 244 Sônia Sin Singer Brugiolo, Luc
- Page 263 and 264: 246 Sônia Sin Singer Brugiolo, Luc
- Page 265 and 266: 248 Sônia Sin Singer Brugiolo, Luc
- Page 267 and 268: 250 Sônia Sin Singer Brugiolo, Luc
- Page 269 and 270: 252 Sônia Sin Singer Brugiolo, Luc
- Page 271 and 272: 254 Sônia Sin Singer Brugiolo, Luc
- Page 273 and 274: 256 C.W. Huck and G.K. Bonn Keyword
- Page 275 and 276: 258 C.W. Huck and G.K. Bonn No addi
- Page 277 and 278: 260 C.W. Huck and G.K. Bonn The rob
- Page 279 and 280: 262 C.W. Huck and G.K. Bonn Validat
- Page 281 and 282: 264 Naphthodianthrones C.W. Huck an
- Page 283 and 284: 266 C.W. Huck and G.K. Bonn Figure
- Page 285 and 286: 268 C.W. Huck and G.K. Bonn Figure
- Page 287 and 288: 270 C.W. Huck and G.K. Bonn hierarc
- Page 289 and 290: 272 C.W. Huck and G.K. Bonn [17] Hu
- Page 292 and 293: In: Medicinal Plants Classification
- Page 294 and 295: What is the Future of Phytotherapy?
- Page 296 and 297: In: Medicinal Plants Classification
- Page 298 and 299: Quality Control of Polysaccharides
- Page 300 and 301:
Quality Control of Polysaccharides
- Page 302 and 303:
Quality Control of Polysaccharides
- Page 304 and 305:
Quality Control of Polysaccharides
- Page 306 and 307:
Quality Control of Polysaccharides
- Page 308 and 309:
Quality Control of Polysaccharides
- Page 310 and 311:
Quality Control of Polysaccharides
- Page 312 and 313:
Origins Targets Neisseria meningiti
- Page 314 and 315:
Quality Control of Polysaccharides
- Page 316 and 317:
Quality Control of Polysaccharides
- Page 318 and 319:
Quality Control of Polysaccharides
- Page 320 and 321:
Quality Control of Polysaccharides
- Page 322 and 323:
Quality Control of Polysaccharides
- Page 324 and 325:
Quality Control of Polysaccharides
- Page 326 and 327:
Quality Control of Polysaccharides
- Page 328 and 329:
Quality Control of Polysaccharides
- Page 330:
Quality Control of Polysaccharides
- Page 333 and 334:
316 Philippe N. Okusa, Caroline Ste
- Page 335 and 336:
318 Philippe N. Okusa, Caroline Ste
- Page 337 and 338:
320 2.7. General Toxicity Tests Phi
- Page 339 and 340:
322 Philippe N. Okusa, Caroline Ste
- Page 341 and 342:
324 Philippe N. Okusa, Caroline Ste
- Page 343 and 344:
326 Philippe N. Okusa, Caroline Ste
- Page 345 and 346:
328 Philippe N. Okusa, Caroline Ste
- Page 347 and 348:
330 Philippe N. Okusa, Caroline Ste
- Page 349 and 350:
332 Philippe N. Okusa, Caroline Ste
- Page 351 and 352:
334 Philippe N. Okusa, Caroline Ste
- Page 353 and 354:
336 Philippe N. Okusa, Caroline Ste
- Page 355 and 356:
338 age-related macular degeneratio
- Page 357 and 358:
340 B lymphocytes, 7 babies, 60, 62
- Page 359 and 360:
342 cell surface, 229 cellular adhe
- Page 361 and 362:
344 cultivation, 75, 76, 83, 85, 14
- Page 363 and 364:
346 embryonic stem cells, 47 emotio
- Page 365 and 366:
348 gas, xiii, 21, 104, 131, 145, 1
- Page 367 and 368:
350 high-performance liquid chromat
- Page 369 and 370:
352 institutions, 84 instruments, 2
- Page 371 and 372:
354 lung, ix, 1, 6, 16, 17, 22, 23,
- Page 373 and 374:
356 mucosa, x, 2, 19, 97, 98, 99, 1
- Page 375 and 376:
358 oxidative, 4, 8, 9, 10, 12, 13,
- Page 377 and 378:
360 polyunsaturated fatty acids, 10
- Page 379 and 380:
362 Reserpine, 328 reservation, 143
- Page 381 and 382:
364 SPT, 135 squamous cell carcinom
- Page 383 and 384:
366 transcription, 8, 35, 152 trans