- Page 2 and 3:
Introduction The methods used to an
- Page 4:
eagents and analytical methods. As
- Page 7 and 8:
5. Officiak Testing Methods and Doc
- Page 9 and 10:
8 6.Goument Foods, etc. III-3.Pharm
- Page 12 and 13:
Chapter I: Basic Knowledge Chapter
- Page 14 and 15:
Chapter I: Basic Knowledge at a tim
- Page 16 and 17:
following formula: Chapter I: Basic
- Page 18 and 19:
[Procedures] Chapter I: Basic Knowl
- Page 20 and 21:
Chapter I: Basic Knowledge electrod
- Page 22 and 23:
! Electrolysis cell Titration syste
- Page 24 and 25:
1. Karl Fischer Reagent SS-Z, SS Ch
- Page 26 and 27:
! Helpful hint - 4 Chapter I: Basic
- Page 28 and 29:
[Characteristics] Chapter I: Basic
- Page 30 and 31:
[Characteristics] [Applications] Ch
- Page 32 and 33:
[Characteristics] [Applications] Ch
- Page 34 and 35:
Chapter I: Basic Knowledge a) No es
- Page 36 and 37:
2. Electrolytes for use with Ketone
- Page 38 and 39:
3. Applicability of Karl Fischer Ti
- Page 40 and 41:
Q9 Chapter I: Basic Knowledge What
- Page 42 and 43:
Chapter I: Basic Knowledge Na2S2O5+
- Page 44 and 45:
Chapter I: Basic Knowledge (3) Sila
- Page 46 and 47:
Chapter I: Basic Knowledge Measurin
- Page 48 and 49:
[Procedures] Chapter I: Basic Knowl
- Page 50 and 51:
Chapter I: Basic Knowledge (3) Usin
- Page 52 and 53:
Q12 Chapter I: Basic Knowledge How
- Page 54 and 55:
Silicon rubber Figure 6: Sampling S
- Page 56 and 57:
Chapter I: Basic Knowledge (2) Syri
- Page 58 and 59:
Chapter I: Basic Knowledge (1) Upri
- Page 60 and 61:
Aquamicron CXU 5. Official Testing
- Page 62 and 63:
2. Japan Pharmaceutical Codex (Revi
- Page 64 and 65:
5. Japan LPG Association Standards
- Page 66 and 67:
Chapter I: Basic Knowledge Part 2:
- Page 68 and 69: Chapter I: Basic Knowledge 6. Impor
- Page 70 and 71: Chapter II: Applications - 1 Chapte
- Page 72 and 73: Chapter II: Applications - 1 Chapte
- Page 74 and 75: Chapter II: Applications - 1 [Examp
- Page 76 and 77: Examples of substances that can be
- Page 78 and 79: Chapter II: Applications - 1 [Examp
- Page 80 and 81: (2) Coulometric titration Reagents
- Page 82 and 83: Examples of substances that can be
- Page 84 and 85: Examples of substances that can be
- Page 86 and 87: 7. Aldehydes Key Points Chapter II:
- Page 88 and 89: Chapter II: Applications - 1 1) Sam
- Page 90 and 91: 8. Organic Acids Key Points Chapter
- Page 92 and 93: (2) Coulometric titration Reagents
- Page 94 and 95: 10. Organic Acid Salts Key Points C
- Page 96 and 97: Chapter II: Applications - 1 [Examp
- Page 98 and 99: Chapter II: Applications - 1 Substa
- Page 100 and 101: Substance pKa Sample quantity (g) C
- Page 102 and 103: (2) Coulometric titration Reagents
- Page 104 and 105: Substance Semicarbazide hydrochlori
- Page 106 and 107: 17. Acid Anhydrides Key Points Chap
- Page 108 and 109: 20. Peroxides Key Points Chapter II
- Page 110 and 111: [Examples of Measurement] (1) Volum
- Page 112 and 113: Chapter II: Applications - 1 1-Phen
- Page 114 and 115: 1. Metals and Simple Substances Key
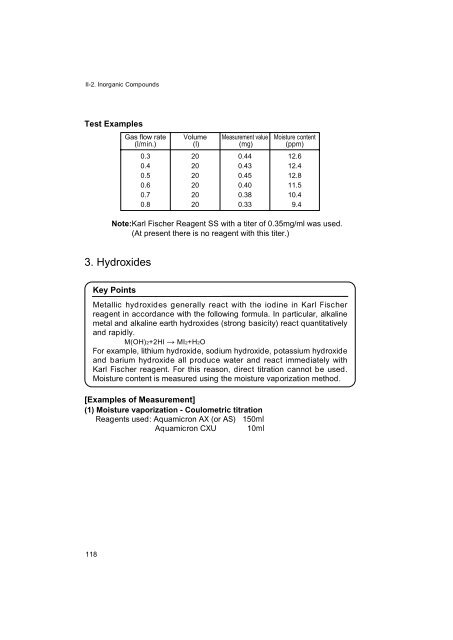
- Page 116 and 117: 2. Inorganic Acids Key Points Chapt
- Page 120 and 121: Substance Calcium hydroxide " Magne
- Page 122 and 123: 5. Halides (halogenides) Key Points
- Page 124 and 125: Substance Potassium chloride " Lith
- Page 126 and 127: 7. Sulfates, Sulfites Key Points Ch
- Page 128 and 129: 8. Other Salts Key Points Chapter I
- Page 130 and 131: 9. Inorganic Gases Key Points Chapt
- Page 132 and 133: Chapter III: Applications - 2 Chapt
- Page 134 and 135: Chapter III: Applications - 2 Chapt
- Page 136 and 137: 2. Agricultural Chemicals Key Point
- Page 138 and 139: [Examples of Measurement] (1) Volum
- Page 140 and 141: Chapter III: Applications - 2 (4) C
- Page 142 and 143: Substance LPG Ethylene Propane Buta
- Page 144 and 145: 6. Petroleum Products Key Points Ch
- Page 146 and 147: Substance Dehydrated solvents [Insu
- Page 148 and 149: 7. Plastics Key Points Chapter III:
- Page 150 and 151: [Examples of Measurement] (1) Moist
- Page 152 and 153: Raw rubber NR SBR BR CR NBR Vulcani
- Page 154 and 155: Substance 10. Dyestuff Intermediate
- Page 156 and 157: (2) Coulometric titration Reagents
- Page 158 and 159: Chapter III: Applications - 2 such
- Page 160 and 161: Chapter III: Applications - 2 (2) M
- Page 162 and 163: Substance Epoxy resins (powder) Iso
- Page 164 and 165: (4) Moisture vaporization - Coulome
- Page 166 and 167: Substance Sample Quantity (g) Measu
- Page 168 and 169:
III-2. Foodstuffs Chapter III: Appl
- Page 170 and 171:
Chapter III: Applications - 2 (2) M
- Page 172 and 173:
2. Cereals and Dried Vegetables Key
- Page 174 and 175:
3. Sugars and Condiments Key Points
- Page 176 and 177:
Chapter III: Applications - 2 [Exam
- Page 178 and 179:
Substance Heating Temperature ( �
- Page 180 and 181:
Chapter III: Applications - 2 (2) M
- Page 182 and 183:
Chapter III: Applications - 2 [Exam
- Page 184 and 185:
Substance Dehydrated solvents Sampl
- Page 186 and 187:
Substance Dehydrated solvents Sampl
- Page 188 and 189:
[Examples of Measurement] (1) Volum
- Page 190 and 191:
2. Natural Products Key Points Subs
- Page 192 and 193:
Chapter IV: List of Karl Fischer Re
- Page 194 and 195:
Chapter IV: List of Karl Fischer Re
- Page 196 and 197:
Chapter IV: List of Karl Fischer Re
- Page 198 and 199:
Chapter IV: List of Karl Fischer Re
- Page 200 and 201:
ChapterV: Summary of Selection Proc
- Page 202 and 203:
Index 202
- Page 204 and 205:
Index B: Bakelite paper ���
- Page 206 and 207:
Index Cyclohexanol �����
- Page 208 and 209:
Index (L-) Histidine ����
- Page 210 and 211:
Index (p-) Nitrophenol ����
- Page 212 and 213:
Index Sodium 5'-guanylate ���
- Page 214 and 215:
214
- Page 216 and 217:
216
- Page 218 and 219:
218 copyright©2004by API Corporati
- Page 220:
Printed in Japan Rev. 3