Development of Karl Fischer Reagents
Development of Karl Fischer Reagents
Development of Karl Fischer Reagents
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
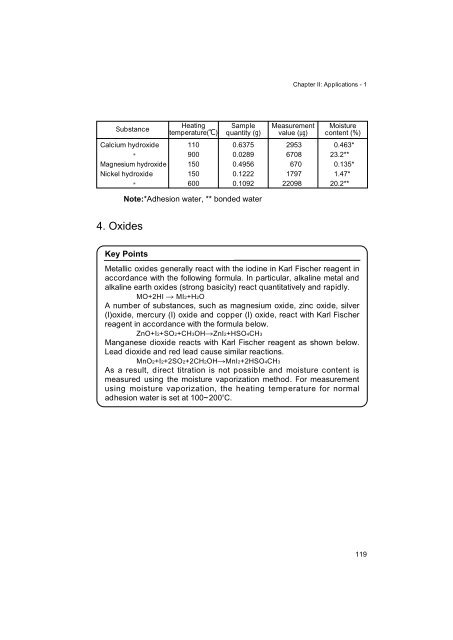
Substance<br />
Calcium hydroxide<br />
"<br />
Magnesium hydroxide<br />
Nickel hydroxide<br />
"<br />
4. Oxides<br />
Key Points<br />
Heating<br />
temperature( � C)<br />
110<br />
900<br />
150<br />
150<br />
600<br />
Sample<br />
quantity (g)<br />
0.6375<br />
0.0289<br />
0.4956<br />
0.1222<br />
0.1092<br />
Note:<br />
*Adhesion water, ** bonded water<br />
Chapter II: Applications - 1<br />
Measurement<br />
value (µg)<br />
2953<br />
6708<br />
670<br />
1797<br />
22098<br />
Moisture<br />
content (%)<br />
0.463*<br />
23.2**<br />
0.135*<br />
1.47*<br />
20.2**<br />
Metallic oxides generally react with the iodine in <strong>Karl</strong> <strong>Fischer</strong> reagent in<br />
accordance with the following formula. In particular, alkaline metal and<br />
alkaline earth oxides (strong basicity) react quantitatively and rapidly.<br />
MO+2HI � MI2+H2O<br />
A number <strong>of</strong> substances, such as magnesium oxide, zinc oxide, silver<br />
(I)oxide, mercury (I) oxide and copper (I) oxide, react with <strong>Karl</strong> <strong>Fischer</strong><br />
reagent in accordance with the formula below.<br />
ZnO+I2+SO2+CH3OH�ZnI2+HSO4CH3<br />
Manganese dioxide reacts with <strong>Karl</strong> <strong>Fischer</strong> reagent as shown below.<br />
Lead dioxide and red lead cause similar reactions.<br />
MnO2+I2+2SO2+2CH2OH�MnI2+2HSO4CH3<br />
As a result, direct titration is not possible and moisture content is<br />
measured using the moisture vaporization method. For measurement<br />
using moisture vaporization, the heating temperature for normal<br />
adhesion water is set at 100~200�C. 119