Development of Karl Fischer Reagents
Development of Karl Fischer Reagents
Development of Karl Fischer Reagents
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
9. Inorganic Gases<br />
Key Points<br />
Chapter II: Applications - 1<br />
The moisture content <strong>of</strong> non-condensing gases, such as nitrogen,<br />
oxygen and hydrogen, is captured by passing the sample gas through<br />
a titration solvent or electrolyte.<br />
<strong>Karl</strong> <strong>Fischer</strong> titration is then carried out. It is necessary, therefore, to<br />
obtain and assemble equipment which includes pipes for the sample<br />
gases and gas meters. Metal pipes should be used at the injection<br />
ports. Pipes made <strong>of</strong> vinyl chloride or other plastics should be avoided<br />
as these absorb atmospheric moisture.<br />
For volumetric titration, an absorption solution is prepared by mixing a<br />
general-use dehydrated solvent with propylene glycol in a ratio <strong>of</strong> 3:1.<br />
For short periods, general-use dehydrated solvent can be used alone.<br />
In such cases, changes in the quantity <strong>of</strong> the titration solvent, caused<br />
by volatilization, must be monitored carefully.<br />
For substances with low moisture content, accurate measurements can<br />
be obtained by using a low-titer <strong>Karl</strong> <strong>Fischer</strong> reagent.<br />
Because <strong>of</strong> the minute amounts <strong>of</strong> moisture in inorganic gases, we<br />
recommend coulometric titration.<br />
[Examples <strong>of</strong> Measurement]<br />
(1) Volumetric titration<br />
Place 150ml <strong>of</strong> dehydrated solvent in a titration flask. Titrate the <strong>Karl</strong> <strong>Fischer</strong><br />
reagent to the end point to remove all moisture. Next pass the sample gas<br />
through the dehydrated solvent in the titration flask at a rate <strong>of</strong> 0.2-0.5l/min.<br />
The flow <strong>of</strong> gas should be measured using a gas meter. Stop the flow <strong>of</strong> gas<br />
(A) after the amount <strong>of</strong> water absorbed into the dehydrated solvent has<br />
reached 5-50mg. Titrate (Bml) immediately using a <strong>Karl</strong> <strong>Fischer</strong> reagent with<br />
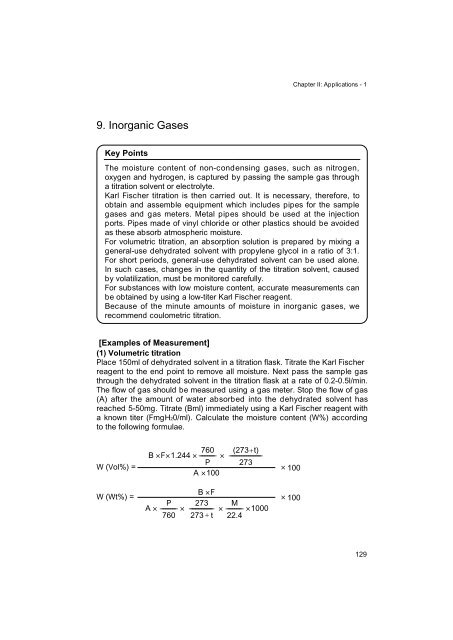
a known titer (FmgH20/ml). Calculate the moisture content (W%) according<br />
to the following formulae.<br />
W (Vol%) =<br />
W (Wt%) =<br />
760<br />
B �F�1.244 �––––––––– �<br />
P<br />
A �100<br />
(273�t)<br />
––––––––––––––––––<br />
273<br />
P<br />
A � ––––––––– �<br />
760<br />
B �F<br />
273<br />
–<br />
273 � t<br />
M<br />
� ––––––––– �1000<br />
22.4<br />
� 100<br />
� 100<br />
129