Histopathology of Seed-Borne Infections - Applied Research Center ...
Histopathology of Seed-Borne Infections - Applied Research Center ...
Histopathology of Seed-Borne Infections - Applied Research Center ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
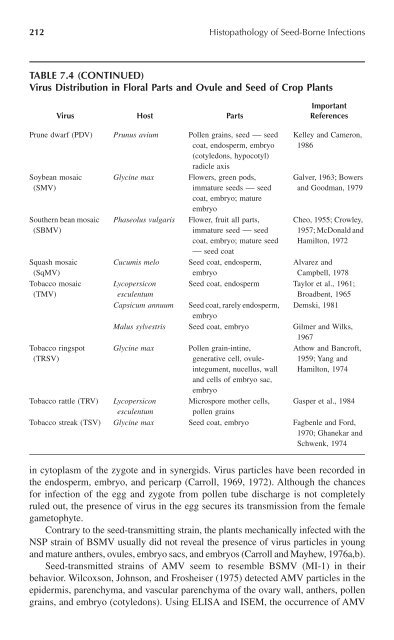
212 <strong>Histopathology</strong> <strong>of</strong> <strong>Seed</strong>-<strong>Borne</strong> <strong>Infections</strong>TABLE 7.4 (CONTINUED)Virus Distribution in Floral Parts and Ovule and <strong>Seed</strong> <strong>of</strong> Crop PlantsVirus Host PartsImportantReferencesPrune dwarf (PDV) Prunus avium Pollen grains, seed — seedcoat, endosperm, embryo(cotyledons, hypocotyl)radicle axisSoybean mosaic(SMV)Southern bean mosaic(SBMV)Squash mosaic(SqMV)Tobacco mosaic(TMV)Tobacco ringspot(TRSV)Glycine maxPhaseolus vulgarisFlowers, green pods,immature seeds — seedcoat, embryo; matureembryoFlower, fruit all parts,immature seed — seedcoat, embryo; mature seed— seed coat<strong>Seed</strong> coat, endosperm,embryoKelley and Cameron,1986Galver, 1963; Bowersand Goodman, 1979Cheo, 1955; Crowley,1957; McDonald andHamilton, 1972Cucumis meloAlvarez andCampbell, 1978Lycopersicon <strong>Seed</strong> coat, endosperm Taylor et al., 1961;esculentumBroadbent, 1965Capsicum annuum <strong>Seed</strong> coat, rarely endosperm, Demski, 1981embryoMalus sylvestris <strong>Seed</strong> coat, embryo Gilmer and Wilks,1967Glycine max Pollen grain-intine,Athow and Bancr<strong>of</strong>t,generative cell, ovuleintegument,1959; Yang andnucellus, wall Hamilton, 1974and cells <strong>of</strong> embryo sac,embryoTobacco rattle (TRV) Lycopersicon Microspore mother cells, Gasper et al., 1984esculentum pollen grainsTobacco streak (TSV) Glycine max <strong>Seed</strong> coat, embryo Fagbenle and Ford,1970; Ghanekar andSchwenk, 1974in cytoplasm <strong>of</strong> the zygote and in synergids. Virus particles have been recorded inthe endosperm, embryo, and pericarp (Carroll, 1969, 1972). Although the chancesfor infection <strong>of</strong> the egg and zygote from pollen tube discharge is not completelyruled out, the presence <strong>of</strong> virus in the egg secures its transmission from the femalegametophyte.Contrary to the seed-transmitting strain, the plants mechanically infected with theNSP strain <strong>of</strong> BSMV usually did not reveal the presence <strong>of</strong> virus particles in youngand mature anthers, ovules, embryo sacs, and embryos (Carroll and Mayhew, 1976a,b).<strong>Seed</strong>-transmitted strains <strong>of</strong> AMV seem to resemble BSMV (MI-1) in theirbehavior. Wilcoxson, Johnson, and Frosheiser (1975) detected AMV particles in theepidermis, parenchyma, and vascular parenchyma <strong>of</strong> the ovary wall, anthers, pollengrains, and embryo (cotyledons). Using ELISA and ISEM, the occurrence <strong>of</strong> AMV