3. FOOD ChEMISTRy & bIOTEChNOLOGy 3.1. Lectures
3. FOOD ChEMISTRy & bIOTEChNOLOGy 3.1. Lectures
3. FOOD ChEMISTRy & bIOTEChNOLOGy 3.1. Lectures
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chem. Listy, 102, s265–s1311 (2008) Food Chemistry & Biotechnology<br />
V a c u u m D i s t i l l a t i o n o f T O M E<br />
Data on amounts of obtained distillates (D1, D2), residues<br />
(Z1, Z2) and weight losses (WL) from 1 st and 2 nd distillation<br />
stage are listed in Table V and VI.<br />
Table V<br />
Vacuum Distillation material balance: 1 st stage<br />
TOME D1 Z1 WL<br />
[g] [g] [wt. %] [g] [wt. %] [wt. %]<br />
2362 845 35.8 1419 60.0 4.2<br />
Table VI<br />
Vacuum distillation material balance: 2 nd stage<br />
TOME D2 Z2 WL<br />
[g] [g] [wt. %] [g] [wt. %] [wt. %]<br />
845.3 712 84.2 101.4 12.0 <strong>3.</strong>8<br />
The prepared amount of TOME was heated and then let<br />
settle. Formed water and unreacted methanol were removed<br />
with a vacuum (molecular) evaporator.<br />
Then 2.5 dm 3 TOME was withdrawn from 3 dm 3 volume<br />
of TOME and distillated at the folowing conditions: pressure<br />
10–50 Pa and temperature 135–140 °C. 955 ml (~ 845 g) of<br />
distillate (D1) was obtained which represented ~ 38 % vol.<br />
(~ 36 % wt.). During this distillation D1 was polluted<br />
by RA.<br />
Destillate D1 955 ml (~ 845 g) was consequently distillated<br />
at pressure 2–20 Pa and temperature 130–135 °C.<br />
Under these conditions, we obtained 835 ml (711.8 g) of distillate<br />
D2, i.e. ~ 87 % vol. from D1, (84 % wt.)<br />
The total yield of these processes: from 2,500 ml TOME<br />
(2,363 g) we obtained 835 ml (711.8 g) which represented<br />
3<strong>3.</strong>4 % vol. (30.1 % wt.).<br />
Acid number parameter of D2 (19.610 mg KOH g –1 ) did<br />
not comply with European standard En 14214 for biodiesel<br />
(stipulating less than 0.5 mg KOH g –1 ), D2 had to undergo<br />
further treatment. D2 included some RA. They were neutralised<br />
and precipitated and D2 (tall oil fatty acids methyl esthers<br />
– TOFAME) after this treatment had acid number 0.3 mg<br />
KOH g –1 . This value fully meets En 14214. However, total<br />
volume of the final product decreased from 835 to 630 ml<br />
(566.6 g) which represented – when comparing to the TOME<br />
amount – 25.2 % vol., (~ 24 % wt.).<br />
The final product prepared in such a mode was subjected<br />
to analyses in laboratory of VÚRUP, which was accredited<br />
for analyses of biofuels. Results are assembled in Table VII.<br />
R e s u l t s O b t a i n e d b y F o u r i e r<br />
T r a n s f o r m I n f r a r e d S p e c t r o m e t r y<br />
( F T I R )<br />
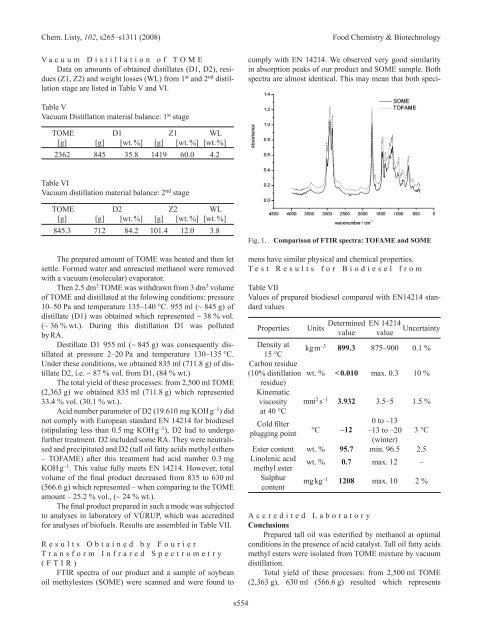
FTIR spectra of our product and a sample of soybean<br />
oil methylesters (SOME) were scanned and were found to<br />
s554<br />
comply with En 14214. We observed very good similarity<br />
in absorption peaks of our product and SOME sample. Both<br />
spectra are almost identical. This may mean that both speci-<br />
Fig. 1. Comparison of FTIR spectra: TOFAME and SOME<br />
mens have similar physical and chemical properties.<br />
T e s t R e s u l t s f o r B i o d i e s e l f r o m<br />
Table VII<br />
Values of prepared biodiesel compared with En14214 standard<br />
values<br />
Properties Units Determined En 14214 Uncertainty<br />
value value<br />
Density at<br />
kg m –3 15 °C<br />
Carbon residue<br />
899.3 875–900 0.1 %<br />
(10% distillation wt. %<br />
residue)<br />
Kinematic<br />
< 0.010 max. 0.3 10 %<br />
mm2 s –1 viscosity<br />
at 40 °C<br />
<strong>3.</strong>932 <strong>3.</strong>5–5 1.5 %<br />
Cold filter<br />
plugging point<br />
°C –12<br />
0 to –13<br />
–13 to –20<br />
(winter)<br />
3 °C<br />
Ester content wt. % 95.7 min. 96.5 2.5<br />
Linolenic acid<br />
methyl ester<br />
wt. % 0.7 max. 12 –<br />
Sulphur<br />
mg kg –1 content<br />
1208 max. 10 2 %<br />
A c c r e d i t e d L a b o r a t o r y<br />
Conclusions<br />
Prepared tall oil was esterified by methanol at optimal<br />
conditions in the presence of acid catalyst. Tall oil fatty acids<br />
methyl esters were isolated from TOME mixture by vacuum<br />
distillation.<br />
Total yield of these processes: from 2,500 ml TOME<br />
(2,363 g), 630 ml (566.6 g) resulted which represents