Prospects of Colloidal Nanocrystals for Electronic - Computer Science
Prospects of Colloidal Nanocrystals for Electronic - Computer Science
Prospects of Colloidal Nanocrystals for Electronic - Computer Science
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
398 Chemical Reviews, 2010, Vol. 110, No. 1 Talapin et al.<br />
3.3. Multicomponent Nanoparticle<br />
Superstructures<br />
Mixing two colloidal solutions <strong>of</strong> NCs with different size<br />
and functionality (e.g., transition metals, semiconductors,<br />
oxides, etc.) can lead to the <strong>for</strong>mation <strong>of</strong> ordered binary<br />
nanoparticle superlattices (BNSLs) (Figures 9-11). 215,220-224<br />
Multicomponent nanoparticle superlattices naturally provide<br />
a much broader range <strong>of</strong> compositions and structures as<br />
compared to the assemblies <strong>of</strong> one type <strong>of</strong> NCs. When<br />
particles <strong>of</strong> different sizes and types are brought together,<br />
they must adjust themselves to space constrains in a certain<br />
way. Many ef<strong>for</strong>ts were made to predict the <strong>for</strong>mation <strong>of</strong> various<br />
ordered binary structures and evaluate their stability. 225-231<br />
In the simplest mechanistic approach based on space filling,<br />
the <strong>for</strong>mation <strong>of</strong> a binary assembly <strong>of</strong> hard (noninteracting)<br />
spheres is expected only if its packing density exceeds the<br />
packing density <strong>of</strong> single-component crystals in fcc or in<br />
hcp structure (∼0.7405). 232 According to this principle, the<br />
key factors determining the structure <strong>of</strong> superlattices are<br />
particle size ratio (γ ) Rsmall/Rlarge) and relative concentrations.<br />
Each structure has its own γ range <strong>of</strong> stability. 232<br />
Taking into account geometrical considerations only, we can<br />
expect the <strong>for</strong>mation <strong>of</strong> superlattices isostructural with NaCl,<br />
NaZn13, and AlB2. Indeed, NaZn13- and AlB2-type assemblies<br />
<strong>of</strong> silica particles were first found in natural Brazilian opals 233<br />
and later grown from latex spheres. 234,235 Packing density in<br />
the stable γ range usually exceeds 0.7405, the packing<br />
density <strong>for</strong> fcc single component lattices. In other words,<br />
the higher is the packing density, the larger is the entropy<br />
gain achieved during crystallization <strong>of</strong> the NC superlattice. 233<br />
Detailed computer simulations showed that the <strong>for</strong>mation <strong>of</strong><br />
NaCl-, AlB2-, and NaZn13-type structures <strong>of</strong> hard spheres<br />
can be driven by entropy alone without any specific energetic<br />
interactions between the particles. 228-230 However, the intrinsic<br />
entropy <strong>of</strong> binary superlattice also affects its stability.<br />
For example, the maximum packing density <strong>of</strong> NaZn13-type<br />
superlattice is slightly below 0.74, whereas the detailed<br />
entropy calculations predict the stability <strong>of</strong> this structure in<br />
a rather broad range <strong>of</strong> γ. 230<br />
Self-assembly <strong>of</strong> two types <strong>of</strong> nanoparticles gives rise to<br />
amazing diversity <strong>of</strong> binary superlattices (Figure 10). Superlattices<br />
with AB, AB2, AB3, AB4, AB5, AB6, and AB13<br />
particle stoichiometry with cubic, hexagonal, tetragonal, and<br />
Figure 9. TEM image <strong>of</strong> (100) projection <strong>of</strong> a binary superlattice<br />
isostructural with AuCu intermetallic compound, self-assembled<br />
from 5.8 nm PbSe and 3.4 nm Ag nanoparticles; inset shows the<br />
same structure under higher magnification.<br />
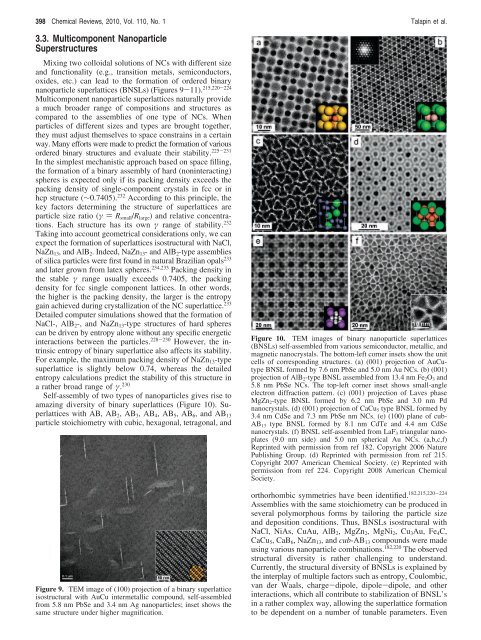
Figure 10. TEM images <strong>of</strong> binary nanoparticle superlattices<br />
(BNSLs) self-assembled from various semiconductor, metallic, and<br />
magnetic nanocrystals. The bottom-left corner insets show the unit<br />
cells <strong>of</strong> corresponding structures. (a) (001) projection <strong>of</strong> AuCutype<br />
BNSL <strong>for</strong>med by 7.6 nm PbSe and 5.0 nm Au NCs. (b) (001)<br />
projection <strong>of</strong> AlB2-type BNSL assembled from 13.4 nm Fe2O3 and<br />
5.8 nm PbSe NCs. The top-left corner inset shows small-angle<br />
electron diffraction pattern. (c) (001) projection <strong>of</strong> Laves phase<br />
MgZn2-type BNSL <strong>for</strong>med by 6.2 nm PbSe and 3.0 nm Pd<br />
nanocrystals. (d) (001) projection <strong>of</strong> CaCu5 type BNSL <strong>for</strong>med by<br />
3.4 nm CdSe and 7.3 nm PbSe nm NCs. (e) (100) plane <strong>of</strong> cub-<br />
AB13 type BNSL <strong>for</strong>med by 8.1 nm CdTe and 4.4 nm CdSe<br />
nanocrystals. (f) BNSL self-assembled from LaF3 triangular nanoplates<br />
(9.0 nm side) and 5.0 nm spherical Au NCs. (a,b,c,f)<br />
Reprinted with permission from ref 182. Copyright 2006 Nature<br />
Publishing Group. (d) Reprinted with permission from ref 215.<br />
Copyright 2007 American Chemical Society. (e) Reprinted with<br />
permission from ref 224. Copyright 2008 American Chemical<br />
Society.<br />
orthorhombic symmetries have been identified. 182,215,220-224<br />
Assemblies with the same stoichiometry can be produced in<br />
several polymorphous <strong>for</strong>ms by tailoring the particle size<br />
and deposition conditions. Thus, BNSLs isostructural with<br />
NaCl, NiAs, CuAu, AlB2, MgZn2, MgNi2, Cu3Au, Fe4C,<br />
CaCu5, CaB6, NaZn13, and cub-AB13 compounds were made<br />
using various nanoparticle combinations. 182,220 The observed<br />
structural diversity is rather challenging to understand.<br />
Currently, the structural diversity <strong>of</strong> BNSLs is explained by<br />
the interplay <strong>of</strong> multiple factors such as entropy, Coulombic,<br />
van der Waals, charge-dipole, dipole-dipole, and other<br />
interactions, which all contribute to stabilization <strong>of</strong> BNSL’s<br />
in a rather complex way, allowing the superlattice <strong>for</strong>mation<br />
to be dependent on a number <strong>of</strong> tunable parameters. Even