Prospects of Colloidal Nanocrystals for Electronic - Computer Science
Prospects of Colloidal Nanocrystals for Electronic - Computer Science
Prospects of Colloidal Nanocrystals for Electronic - Computer Science
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Colloidal</strong> <strong>Nanocrystals</strong> in <strong>Electronic</strong> Applications Chemical Reviews, 2010, Vol. 110, No. 1 431<br />
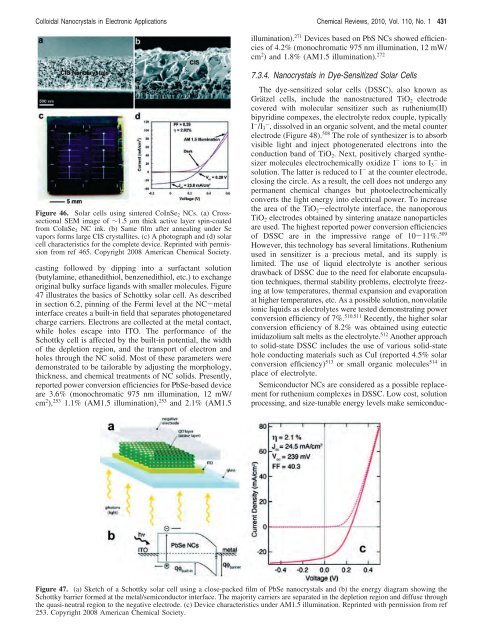
Figure 46. Solar cells using sintered CoInSe2 NCs. (a) Crosssectional<br />
SEM image <strong>of</strong> ∼1.5 µm thick active layer spin-coated<br />
from CoInSe2 NC ink. (b) Same film after annealing under Se<br />
vapors <strong>for</strong>ms large CIS crystallites. (c) A photograph and (d) solar<br />
cell characteristics <strong>for</strong> the complete device. Reprinted with permission<br />
from ref 465. Copyright 2008 American Chemical Society.<br />
casting followed by dipping into a surfactant solution<br />
(butylamine, ethanedithiol, benzenedithiol, etc.) to exchange<br />
original bulky surface ligands with smaller molecules. Figure<br />
47 illustrates the basics <strong>of</strong> Schottky solar cell. As described<br />
in section 6.2, pinning <strong>of</strong> the Fermi level at the NC-metal<br />
interface creates a built-in field that separates photogenetared<br />
charge carriers. Electrons are collected at the metal contact,<br />
while holes escape into ITO. The per<strong>for</strong>mance <strong>of</strong> the<br />
Schottky cell is affected by the built-in potential, the width<br />
<strong>of</strong> the depletion region, and the transport <strong>of</strong> electron and<br />
holes through the NC solid. Most <strong>of</strong> these parameters were<br />
demonstrated to be tailorable by adjusting the morphology,<br />
thickness, and chemical treatments <strong>of</strong> NC solids. Presently,<br />
reported power conversion efficiencies <strong>for</strong> PbSe-based device<br />
are 3.6% (monochromatic 975 nm illumination, 12 mW/<br />
cm 2 ), 253 1.1% (AM1.5 illumination), 253 and 2.1% (AM1.5<br />
illumination). 271 Devices based on PbS NCs showed efficiencies<br />
<strong>of</strong> 4.2% (monochromatic 975 nm illumination, 12 mW/<br />
cm 2 ) and 1.8% (AM1.5 illumination). 272<br />
7.3.4. <strong>Nanocrystals</strong> in Dye-Sensitized Solar Cells<br />
The dye-sensitized solar cells (DSSC), also known as<br />
Grätzel cells, include the nanostructured TiO2 electrode<br />
covered with molecular sensitizer such as ruthenium(II)<br />
bipyridine compexes, the electrolyte redox couple, typically<br />
I - /I3 - , dissolved in an organic solvent, and the metal counter<br />
electrode (Figure 48). 508 The role <strong>of</strong> synthesizer is to absorb<br />
visible light and inject photogenerated electrons into the<br />
conduction band <strong>of</strong> TiO2. Next, positively charged synthesizer<br />
molecules electrochemically oxidize I - ions to I3 - in<br />
solution. The latter is reduced to I - at the counter electrode,<br />
closing the circle. As a result, the cell does not undergo any<br />
permanent chemical changes but photoelectrochemically<br />
converts the light energy into electrical power. To increase<br />
the area <strong>of</strong> the TiO2-electrolyte interface, the nanoporous<br />
TiO2 electrodes obtained by sintering anataze nanoparticles<br />
are used. The highest reported power conversion efficiencies<br />
<strong>of</strong> DSSC are in the impressive range <strong>of</strong> 10-11%. 509<br />
However, this technology has several limitations. Ruthenium<br />
used in sensitizer is a precious metal, and its supply is<br />
limited. The use <strong>of</strong> liquid electrolyte is another serious<br />
drawback <strong>of</strong> DSSC due to the need <strong>for</strong> elaborate encapsulation<br />
techniques, thermal stability problems, electrolyte freezing<br />
at low temperatures, thermal expansion and evaporation<br />
at higher temperatures, etc. As a possible solution, nonvolatile<br />
ionic liquids as electrolytes were tested demonstrating power<br />
conversion efficiency <strong>of</strong> 7%. 510,511 Recently, the higher solar<br />
conversion efficiency <strong>of</strong> 8.2% was obtained using eutectic<br />
imidazolium salt melts as the electrolyte. 512 Another approach<br />
to solid-state DSSC includes the use <strong>of</strong> various solid-state<br />
hole conducting materials such as CuI (reported 4.5% solar<br />
conversion efficiency) 513 or small organic molecules 514 in<br />
place <strong>of</strong> electrolyte.<br />
Semiconductor NCs are considered as a possible replacement<br />
<strong>for</strong> ruthenium complexes in DSSC. Low cost, solution<br />
processing, and size-tunable energy levels make semiconduc-<br />
Figure 47. (a) Sketch <strong>of</strong> a Schottky solar cell using a close-packed film <strong>of</strong> PbSe nanocrystals and (b) the energy diagram showing the<br />
Schottky barrier <strong>for</strong>med at the metal/semiconductor interface. The majority carriers are separated in the depletion region and diffuse through<br />
the quasi-neutral region to the negative electrode. (c) Device characteristics under AM1.5 illumination. Reprinted with permission from ref<br />
253. Copyright 2008 American Chemical Society.