Prospects of Colloidal Nanocrystals for Electronic - Computer Science
Prospects of Colloidal Nanocrystals for Electronic - Computer Science
Prospects of Colloidal Nanocrystals for Electronic - Computer Science
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
394 Chemical Reviews, 2010, Vol. 110, No. 1 Talapin et al.<br />
faces. 106 Obtained aerogels retain a much stronger resemblance<br />
to their original gel network structure than do<br />
xerogels. For more details on sol-gel synthesis, we address<br />
the reader to several recent review articles. 105,107-111<br />
Brock et al. reported the synthesis <strong>of</strong> xerogels and aerogels<br />
assembled from CdS, ZnS, PbS, CdSe, and CdSe/ZnS core/<br />
shell nanoparticles. 112,113 Primary nanoparticles were aggregated<br />
to the wet gels and then dried under ambient<br />
conditions or in supercritical CO2 to produce nanoparticle<br />
xerogels and aerogels, respectively.<br />
2.2. Nanoparticle Shape and Morphology<br />
Engineering<br />
In analogy with bulk crystals, the nanoparticles are<br />
terminated by facets that expose different crystallographic<br />
planes. Selective adhesion <strong>of</strong> surfactant molecules allows <strong>for</strong><br />
tuning the growth kinetics <strong>of</strong> different crystal facets and<br />
tailoring the NC shape from nearly spherical to highly<br />
anisotropic 30 (Figure 4). Strong binding <strong>of</strong> capping molecules<br />
suppresses the growth <strong>of</strong> certain facets, leading to a variety<br />
<strong>of</strong> NC shapes. The multicomponent mixtures <strong>of</strong> stabilizing<br />
agents are <strong>of</strong>ten employed to provide the difference in growth<br />
rate in different crystallographic directions. For example,<br />
depending on the length <strong>of</strong> alkyl chain and concentration <strong>of</strong><br />
alkylphosphonic acid and the heating regime, the rod-,<br />
arrow-, rise-, teardrop-, and tetrapod-shaped CdSe NCs can<br />
be synthesized. 4,32 Possible nanoparticle shapes are determined<br />
by symmetry <strong>of</strong> underlying crystal lattice; <strong>for</strong> example,<br />
PbSe NCs with rocksalt atomic lattice can be synthesized in<br />
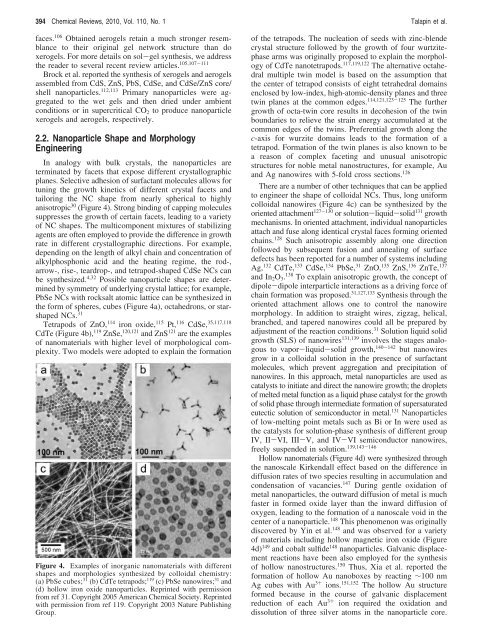
the <strong>for</strong>m <strong>of</strong> spheres, cubes (Figure 4a), octahedrons, or starshaped<br />
NCs. 31<br />
Tetrapods <strong>of</strong> ZnO, 114 iron oxide, 115 Pt, 116 CdSe, 35,117,118<br />
CdTe (Figure 4b), 119 ZnSe, 120,121 and ZnS 121 are the examples<br />
<strong>of</strong> nanomaterials with higher level <strong>of</strong> morphological complexity.<br />
Two models were adopted to explain the <strong>for</strong>mation<br />
Figure 4. Examples <strong>of</strong> inorganic nanomaterials with different<br />
shapes and morphologies synthesized by colloidal chemistry:<br />
(a) PbSe cubes; 31 (b) CdTe tetrapods; 119 (c) PbSe nanowires; 31 and<br />
(d) hollow iron oxide nanoparticles. Reprinted with permission<br />
from ref 31. Copyright 2005 American Chemical Society. Reprinted<br />
with permission from ref 119. Copyright 2003 Nature Publishing<br />
Group.<br />
<strong>of</strong> the tetrapods. The nucleation <strong>of</strong> seeds with zinc-blende<br />
crystal structure followed by the growth <strong>of</strong> four wurtzitephase<br />
arms was originally proposed to explain the morphology<br />
<strong>of</strong> CdTe nanotetrapods. 117,119,122 The alternative octahedral<br />
multiple twin model is based on the assumption that<br />
the center <strong>of</strong> tetrapod consists <strong>of</strong> eight tetrahedral domains<br />
enclosed by low-index, high-atomic-density planes and three<br />
twin planes at the common edges. 114,121,123-125 The further<br />
growth <strong>of</strong> octa-twin core results in decohesion <strong>of</strong> the twin<br />
boundaries to relieve the strain energy accumulated at the<br />
common edges <strong>of</strong> the twins. Preferential growth along the<br />
c-axis <strong>for</strong> wurzite domains leads to the <strong>for</strong>mation <strong>of</strong> a<br />
tetrapod. Formation <strong>of</strong> the twin planes is also known to be<br />
a reason <strong>of</strong> complex faceting and unusual anisotropic<br />
structures <strong>for</strong> noble metal nanostructures, <strong>for</strong> example, Au<br />
and Ag nanowires with 5-fold cross sections. 126<br />
There are a number <strong>of</strong> other techniques that can be applied<br />
to engineer the shape <strong>of</strong> colloidal NCs. Thus, long uni<strong>for</strong>m<br />
colloidal nanowires (Figure 4c) can be synthesized by the<br />
oriented attachment 127-130 or solution-liquid-solid 131 growth<br />
mechanisms. In oriented attachment, individual nanoparticles<br />
attach and fuse along identical crystal faces <strong>for</strong>ming oriented<br />
chains. 128 Such anisotropic assembly along one direction<br />
followed by subsequent fusion and annealing <strong>of</strong> surface<br />
defects has been reported <strong>for</strong> a number <strong>of</strong> systems including<br />
Ag, 132 CdTe, 133 CdSe, 134 PbSe, 31 ZnO, 135 ZnS, 136 ZnTe, 137<br />
and In2O3. 138 To explain anisotropic growth, the concept <strong>of</strong><br />
dipole-dipole interparticle interactions as a driving <strong>for</strong>ce <strong>of</strong><br />
chain <strong>for</strong>mation was proposed. 31,127,133 Synthesis through the<br />
oriented attachment allows one to control the nanowire<br />
morphology. In addition to straight wires, zigzag, helical,<br />
branched, and tapered nanowires could all be prepared by<br />
adjustment <strong>of</strong> the reaction conditions. 31 Solution liquid solid<br />
growth (SLS) <strong>of</strong> nanowires 131,139 involves the stages analogous<br />
to vapor-liquid-solid growth, 140-142 but nanowires<br />
grow in a colloidal solution in the presence <strong>of</strong> surfactant<br />
molecules, which prevent aggregation and precipitation <strong>of</strong><br />
nanowires. In this approach, metal nanoparticles are used as<br />
catalysts to initiate and direct the nanowire growth; the droplets<br />
<strong>of</strong> melted metal function as a liquid phase catalyst <strong>for</strong> the growth<br />
<strong>of</strong> solid phase through intermediate <strong>for</strong>mation <strong>of</strong> supersaturated<br />
eutectic solution <strong>of</strong> semiconductor in metal. 131 Nanoparticles<br />
<strong>of</strong> low-melting point metals such as Bi or In were used as<br />
the catalysts <strong>for</strong> solution-phase synthesis <strong>of</strong> different group<br />
IV, II-VI, III-V, and IV-VI semiconductor nanowires,<br />
freely suspended in solution. 139,143-146<br />
Hollow nanomaterials (Figure 4d) were synthesized through<br />
the nanoscale Kirkendall effect based on the difference in<br />
diffusion rates <strong>of</strong> two species resulting in accumulation and<br />
condensation <strong>of</strong> vacancies. 147 During gentle oxidation <strong>of</strong><br />
metal nanoparticles, the outward diffusion <strong>of</strong> metal is much<br />
faster in <strong>for</strong>med oxide layer than the inward diffusion <strong>of</strong><br />
oxygen, leading to the <strong>for</strong>mation <strong>of</strong> a nanoscale void in the<br />
center <strong>of</strong> a nanoparticle. 148 This phenomenon was originally<br />
discovered by Yin et al. 148 and was observed <strong>for</strong> a variety<br />
<strong>of</strong> materials including hollow magnetic iron oxide (Figure<br />
4d) 149 and cobalt sulfide 148 nanoparticles. Galvanic displacement<br />
reactions have been also employed <strong>for</strong> the synthesis<br />
<strong>of</strong> hollow nanostructures. 150 Thus, Xia et al. reported the<br />
<strong>for</strong>mation <strong>of</strong> hollow Au nanoboxes by reacting ∼100 nm<br />
Ag cubes with Au 3+ ions. 151,152 The hollow Au structure<br />
<strong>for</strong>med because in the course <strong>of</strong> galvanic displacement<br />
reduction <strong>of</strong> each Au 3+ ion required the oxidation and<br />
dissolution <strong>of</strong> three silver atoms in the nanoparticle core.