1 (US Patent No. US D560281 (S1); US D5601344 (S1); US ...
1 (US Patent No. US D560281 (S1); US D5601344 (S1); US ...
1 (US Patent No. US D560281 (S1); US D5601344 (S1); US ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
9. Failure to bring specimens and reagents to room temperature (20-25 C) before testing may decrease assay sensitivity.<br />
10. Influenza virus antigens are relatively unstable. Care should be taken to store samples as indicated in this document.<br />
11. Performance characteristics for influenza A were established when influenza A/H3 and A/H1 were the predominant<br />
influenza A viruses in circulation. When other influenza A viruses are emerging, performance characteristics may vary.<br />
12. If infection with a novel influenza A virus is suspected, based on current clinical and epidemiological screening criteria<br />
recommended by public health authorities, specimens should be collected with appropriate infection control<br />
precautions for novel virulent influenza viruses and sent to state or local public health departments for testing. Viral<br />
culture should not be attempted in these cases unless a Biosafety Level 3+ facility is available to receive and<br />
culture specimens. Some patient specimens contain infectious agents other than influenza virus; therefore all patient<br />
specimens should be handled and disposed of as if they are biologically hazardous.<br />
13. Meridian’s Flu/RSV Positive Control (sold as an adjunct reagent) contains inactivated influenza A, influenza B and RSV<br />
viruses and should be handled as if it were potentially infectious. This reagent contains 0.094% sodium azide. Sodium<br />
azide is a skin irritant. Avoid skin contact. Disposal of reagents containing sodium azide into drains consisting of lead<br />
or copper plumbing can result in the formation of explosive metal oxides. Eliminate build-up of oxides by flushing<br />
drains with large volumes of water during disposal.<br />
14. Swab samples can be transported in 0.5 mL to 3.0 mL of an approved transport media. Stronger positive reactions<br />
may be obtained if the transport media volume is 0.5 mL to 1.5 mL.<br />
15. Sample Diluent must be added to the Conjugate Tube within one minute after removing the cap from the tube.<br />
PROCEDURAL NOTES<br />
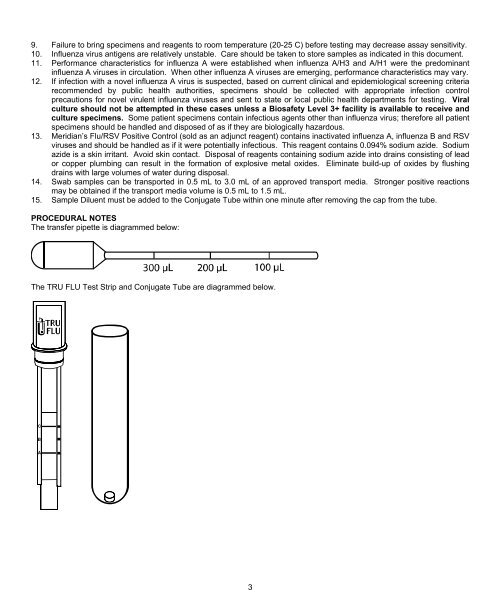
The transfer pipette is diagrammed below:<br />
The TRU FLU Test Strip and Conjugate Tube are diagrammed below.<br />
3