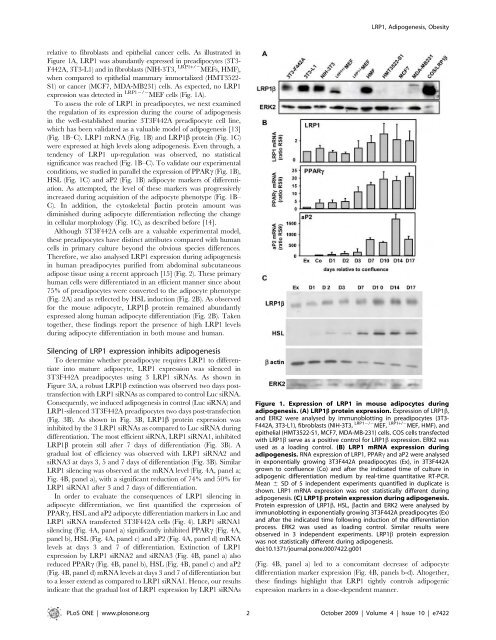
LRP1, Adipogenesis, Obesityre<strong>la</strong>tive to fibrob<strong>la</strong>sts and epithelial cancer cells. As illustrated inFigure 1A, LRP1 was abundantly expressed in preadipocytes (3T3-F442A, 3T3-L1) and in fibrob<strong>la</strong>sts (NIH-3T3, LRP1+/2 MEFs, HMF),when compared to epithelial mammary immortalized (HMT3522-S1) or cancer (MCF7, MDA-MB231) cells. As expected, no LRP1expression was d<strong>et</strong>ected in LRP12/2 MEF cells (Fig. 1A).To assess the role of LRP1 in preadipocytes, we next examinedthe regu<strong>la</strong>tion of its expression <strong>du</strong>ring the course of adipogenesisin the well-established murine 3T3F442A preadipocyte cell line,which has been validated as a valuable mo<strong>de</strong>l of adipogenesis [13](Fig. 1B–C). LRP1 mRNA (Fig. 1B) and LRP1b protein (Fig. 1C)were expressed at high levels along adipogenesis. Even through, aten<strong>de</strong>ncy of LRP1 up-regu<strong>la</strong>tion was observed, no statisticalsignificance was reached (Fig. 1B–C). To validate our experimentalconditions, we studied in parallel the expression of PPARc (Fig. 1B),HSL (Fig. 1C) and aP2 (Fig. 1B) adipocyte markers of differentiation.As attempted, the level of these markers was progressivelyincreased <strong>du</strong>ring acquisition of the adipocyte phenotype (Fig. 1B–C). In addition, the cytoskel<strong>et</strong>al bactin protein amount wasdiminished <strong>du</strong>ring adipocyte differentiation reflecting the changein cellu<strong>la</strong>r morphology (Fig. 1C), as <strong>de</strong>scribed before [14].Although 3T3F442A cells are a valuable experimental mo<strong>de</strong>l,these preadipocytes have distinct attributes compared with humancells in primary culture beyond the obvious species differences.Therefore, we also analysed LRP1 expression <strong>du</strong>ring adipogenesisin human preadipocytes purified from abdominal subcutaneousadipose tissue using a recent approach [15] (Fig. 2). These primaryhuman cells were differentiated in an efficient manner since about75% of preadipocytes were converted to the adipocyte phenotype(Fig. 2A) and as reflected by HSL in<strong>du</strong>ction (Fig. 2B). As observedfor the mouse adipocyte, LRP1b protein remained abundantlyexpressed along human adipocyte differentiation (Fig. 2B). Takentog<strong>et</strong>her, these findings report the presence of high LRP1 levels<strong>du</strong>ring adipocyte differentiation in both mouse and human.Silencing of LRP1 expression inhibits adipogenesisTo d<strong>et</strong>ermine wh<strong>et</strong>her preadipocyte requires LRP1 to differentiateinto mature adipocyte, LRP1 expression was silenced in3T3F442A preadipocytes using 3 LRP1 siRNAs. As shown inFigure 3A, a robust LRP1b extinction was observed two days posttransfectionwith LRP1 siRNAs as compared to control Luc siRNA.Consequently, we in<strong>du</strong>ced adipogenesis in control (Luc siRNA) andLRP1-silenced 3T3F442A preadipocytes two days post-transfection(Fig. 3B). As shown in Fig. 3B, LRP1b protein expression wasinhibited by the 3 LRP1 siRNAs as compared to Luc siRNA <strong>du</strong>ringdifferentiation. The most efficient siRNA, LRP1 siRNA1, inhibitedLRP1b protein still after 7 days of differentiation (Fig. 3B). Agra<strong>du</strong>al lost of efficiency was observed with LRP1 siRNA2 andsiRNA3 at days 3, 5 and 7 days of differentiation (Fig. 3B). Simi<strong>la</strong>rLRP1 silencing was observed at the mRNA level (Fig. 4A, panel a;Fig. 4B, panel a), with a significant re<strong>du</strong>ction of 74% and 50% forLRP1 siRNA1 after 3 and 7 days of differentiation.In or<strong>de</strong>r to evaluate the consequences of LRP1 silencing inadipocyte differentiation, we first quantified the expression ofPPARc, HSL and aP2 adipocyte differentiation markers in Luc andLRP1 siRNA transfected 3T3F442A cells (Fig. 4). LRP1 siRNA1silencing (Fig. 4A, panel a) significantly inhibited PPARc (Fig. 4A,panel b), HSL (Fig. 4A, panel c) and aP2 (Fig. 4A, panel d) mRNAlevels at days 3 and 7 of differentiation. Extinction of LRP1expression by LRP1 siRNA2 and siRNA3 (Fig. 4B, panel a) alsore<strong>du</strong>ced PPARc (Fig. 4B, panel b), HSL (Fig. 4B, panel c) and aP2(Fig. 4B, panel d) mRNA levels at days 3 and 7 of differentiation butto a lesser extend as compared to LRP1 siRNA1. Hence, our resultsindicate that the gra<strong>du</strong>al lost of LRP1 expression by LRP1 siRNAsFigure 1. Expression of LRP1 in mouse adipocytes <strong>du</strong>ringadipogenesis. (A) LRP1b protein expression. Expression of LRP1b,and ERK2 were analysed by immunoblotting in preadipocytes (3T3-F442A, 3T3-L1), fibrob<strong>la</strong>sts (NIH-3T3, LRP12/2 MEF, LRP1+/2 MEF, HMF), an<strong>de</strong>pithelial (HMT3522-S1, MCF7, MDA-MB-231) cells. COS cells transfectedwith LRP1b serve as a positive control for LRP1b expression. ERK2 wasused as a loading control. (B) LRP1 mRNA expression <strong>du</strong>ringadipogenesis. RNA expression of LRP1, PPARc and aP2 were analysedin exponentially growing 3T3F442A preadipocytes (Ex), in 3T3F442Agrown to confluence (Co) and after the indicated time of culture inadipogenic differentiation medium by real-time quantitative RT-PCR.Mean 6 SD of 5 in<strong>de</strong>pen<strong>de</strong>nt experiments quantified in <strong>du</strong>plicate isshown. LRP1 mRNA expression was not statistically different <strong>du</strong>ringadipogenesis. (C) LRP1b protein expression <strong>du</strong>ring adipogenesis.Protein expression of LRP1b, HSL, bactin and ERK2 were analysed byimmunoblotting in exponentially growing 3T3F442A preadipocytes (Ex)and after the indicated time following in<strong>du</strong>ction of the differentiationprocess. ERK2 was used as loading control. Simi<strong>la</strong>r results wereobserved in 3 in<strong>de</strong>pen<strong>de</strong>nt experiments. LRP1b protein expressionwas not statistically different <strong>du</strong>ring adipogenesis.doi:10.1371/journal.pone.0007422.g001(Fig. 4B, panel a) led to a concomitant <strong>de</strong>crease of adipocytedifferentiation marker expression (Fig. 4B, panels b-d). Altog<strong>et</strong>her,these findings highlight that LRP1 tightly controls adipogenicexpression markers in a dose-<strong>de</strong>pen<strong>de</strong>nt manner.PLoS ONE | www.plosone.org 2 October 2009 | Volume 4 | Issue 10 | e7422
LRP1, Adipogenesis, ObesityFigure 2. Expression of LRP1 <strong>du</strong>ring adipogenesis in human. (A) Micrographs of human adipocytes. Human preadipocytes iso<strong>la</strong>ted fromsubcutaneous adipose tissue digested with col<strong>la</strong>genase and separated from the stromal vascu<strong>la</strong>r fraction were grown for 0, 3, 7 and 14 days in thepresence of the adipogenic medium as illustrated in the <strong>micro</strong>graphs. A representative experiment is shown. (B) LRP1b protein expression<strong>du</strong>ring adipogenesis. Protein expression of LRP1b and HSL was analysed by immunoblotting after the indicated time following in<strong>du</strong>ction of thedifferentiation process. NS, non specific band showing sample loading. Two in<strong>de</strong>pen<strong>de</strong>nt experiments (left and right panels) are presented.doi:10.1371/journal.pone.0007422.g002Then, we analysed the cellu<strong>la</strong>r lipid levels in adipocytes silencedor not for LRP1 (Fig. 5). In<strong>de</strong>ed, the most obvious feature ofadipocytes is the synthesis and storage of triglyceri<strong>de</strong>s in lipiddropl<strong>et</strong>s and therefore the gra<strong>du</strong>al appearance and growth of lipiddropl<strong>et</strong>s are characteristic for adipocyte precursor cells un<strong>de</strong>rgoingadipogenic differentiation. The presence of these neutral lipids wasd<strong>et</strong>ected by oil red O staining (Fig. 5A–B). As illustrated in Fig. 5A,oil red O staining after 7 days of differentiation revealed thatextinction of LRP1 expression by siRNA1 strongly <strong>de</strong>creasedneutral lipid dropl<strong>et</strong> formation and/or accumu<strong>la</strong>tion. Microscopicanalysis at day 3 and 7 of differentiation illustrated that, asattempted, Luc siRNA-transfected 3T3F442A cells accumu<strong>la</strong>tednumerous <strong>la</strong>rge lipid dropl<strong>et</strong>s and adopted a non adherent roundmorphology characteristic of mature adipocytes (Fig. 5B, panels aand c). By contrast, in 3T3F442A cells silenced with LRP1siRNA1, the lipid dropl<strong>et</strong> size and number were strongly re<strong>du</strong>ced(Fig. 5B, panels b and d). Moreover, cells kept the morphologicalfeature of adherent fibrob<strong>la</strong>stic cells, suggesting that preadipocytedifferentiation process did not occur in the absence of LRP1.Quantification of lipids afterwards <strong>de</strong>monstrated that silencing ofLRP1 with LRP1 siRNA1 significantly <strong>de</strong>creased lipid contentlevels by 7 fold at day 7 of differentiation (Fig. 5C, panel a). LRP1siRNA2 and siRNA3 also lowered the lipid content but to a lesserextend as compared to LRP1 siRNA1 (Fig. 5C, panel a). Simi<strong>la</strong>rresults were obtained by quantifying triglyceri<strong>de</strong>s (Fig. 5C, panelb). These results reveal that LRP1 silencing in preadipocytesinhibits adipogenesis, leading to lipid-<strong>de</strong>pl<strong>et</strong>ed cells.We finally studied the effects of silencing LRP1 in preadipocyteon lipolysis. In<strong>de</strong>ed, only mature adipocytes possess the compl<strong>et</strong>eapparatus for lipolysis. Our results indicating a significant <strong>de</strong>creaseof HSL expression in LRP1 silenced cells (Fig. 4), we, therefore,advocate that lipolysis should be absent in LRP1-silencedpreadipocytes. An alternative could be that LRP1 silenced cellshave an increased lipolysis, leading to lipid <strong>de</strong>pl<strong>et</strong>ed cells.However, our results revealed that glycerol release over 18 hwas significantly re<strong>du</strong>ced in LRP1 silenced cells, indicatinginhibition of basal lipolysis (Fig. 6A). Moreover, glycerol andNEFA released in the media after isoproterenol treatment werealso significantly <strong>de</strong>creased in LRP1 silenced cells, evi<strong>de</strong>ncinginhibition of in<strong>du</strong>ced-lipolysis. Therefore, extinction of LRP1 inpreadipocytes abolishes expression of adipocyte differentiationmarkers and leads to lipid-<strong>de</strong>pl<strong>et</strong>ed cells inept to in<strong>du</strong>ce lipolysis.LRP1 expression is up-regu<strong>la</strong>ted in human and mouseobese adipose tissuesObesity is characterized by the increase of intracellu<strong>la</strong>r lipidaccumu<strong>la</strong>tion which shows a significant corre<strong>la</strong>tion with adipocytedifferentiation. LRP1 mRNA expression was investigated in humanintra-abdominal visceral adipose tissue (VAT) from lean (42.764.5year old, BMI: 23.163.3 kg/m 2 ) and obese (44.561.8 year old,BMI: 47.661.3 kg/m 2 ) human (Fig. 7). Interestingly, LRP1 mRNAwas significantly increased in human obese adipose tissue (Fig. 7A).To assess wh<strong>et</strong>her this LRP1 up-regu<strong>la</strong>tion was a generalcharacteristic of obese adipocytes, we then analysed LRP1 mRNAPLoS ONE | www.plosone.org 3 October 2009 | Volume 4 | Issue 10 | e7422
- Page 1 and 2:
Université Montpellier I UFR Méd
- Page 3 and 4:
Un grand merci à tous mes collabor
- Page 5 and 6:
TitleBreast cancer and tumoral micr
- Page 7 and 8:
Cancer du sein et micro-environneme
- Page 9 and 10:
C. PRESENTATION DU TRAVAIL DE THESE
- Page 11 and 12:
But de la thèseLa cathepsine-D (ca
- Page 13 and 14:
I. Le tissu adipeux1) Généralité
- Page 15 and 16:
(Adenosine triphosphate). Cette pro
- Page 18 and 19:
Cette synthèse de novo a lieu dans
- Page 20 and 21:
ABFigure 6 : Schéma de la oxydati
- Page 22 and 23:
d. Ladipocyte : une cellule sécré
- Page 24 and 25:
3) Ladipogenèsea. Les différentes
- Page 26 and 27:
. Les facteurs de transcriptionLa d
- Page 28 and 29:
Lexpression de C/EBP est quant à e
- Page 30 and 31:
adipeux. Les analyses histologiques
- Page 32 and 33:
adipocyteFigure 10 : Schéma repré
- Page 34 and 35:
II. La cathepsine-D1) Synthèse, ma
- Page 36 and 37:
les cath-B et L, en une forme matur
- Page 38 and 39:
Il existe deux RM6P : le RM6P/IGFII
- Page 40 and 41:
2) Fonctions de la cath-Da. Dans la
- Page 42 and 43:
. Dans les pathologiesEn plus de se
- Page 44 and 45:
c. Rôles et mécanismes daction da
- Page 46 and 47:
carcinome de prostate serait respon
- Page 48 and 49: La cath-D joue donc un rôle import
- Page 50 and 51: Une fois le marquage des peptides e
- Page 52 and 53: Afin de réaliser ce projet, nous a
- Page 54 and 55: III. Le récepteur LRP11) Organisat
- Page 56 and 57: 2) Trafic intra-cellulaireComme le
- Page 58 and 59: LRP1αLRP1Membrane associatedprotea
- Page 60 and 61: Tableau 3 : Ligands connus du LRP1(
- Page 62 and 63: I. Etude du rôle de la cathepsine
- Page 64 and 65: Cathepsin-D, a key protease in brea
- Page 66 and 67: IntroductionConsumption of meals ri
- Page 68 and 69: (Fig. 1A, panel a). This differenti
- Page 70 and 71: differentiated adipocyte (Fig. 4B).
- Page 72 and 73: DiscussionOur results demonstrate t
- Page 74 and 75: from normal and peri-al breast adip
- Page 76 and 77: mouse RS9 (sens 5CGGCCCGGGAGCTGTTGA
- Page 78 and 79: agreement of local ethic committee.
- Page 80 and 81: Statistical analysis. Results are e
- Page 82 and 83: Loncarek J, Freiss G, Vignon F and
- Page 84 and 85: Aa3000 *b4000*B8000**cath-D mRNA(ar
- Page 86 and 87: ABcath-D mRNA(ratio RS9)PPARg mRNA(
- Page 88 and 89: A D3 D7 D14D0BaD0 D3 D7 D14D0 D3 D7
- Page 90 and 91: AshLucshcath-DF442A C34 C37 A4 D10c
- Page 92 and 93: AF442-AC34C37A4D10BF442-AC34C37A4D1
- Page 94 and 95: II. Etude du rôle du LRP1 dans les
- Page 96 and 97: 2) Article 2:LRP1 receptor controls
- Page 100 and 101: LRP1, Adipogenesis, ObesityFigure 3
- Page 102 and 103: LRP1, Adipogenesis, ObesityFigure 5
- Page 104 and 105: LRP1, Adipogenesis, ObesitySome ins
- Page 106 and 107: LRP1, Adipogenesis, ObesityLipolysi
- Page 108 and 109: CONCLUSIONLa cathepsine D (cath-D)
- Page 110 and 111: la souris obèses. Enfin, linhibiti
- Page 112 and 113: carcinogénique sont encore mal con
- Page 114 and 115: Ce travail de thèse a étudié, po
- Page 116 and 117: REFERENCESAhima, R. S. (2006). Adip
- Page 118 and 119: implicates them as antigen presenti
- Page 120 and 121: Cataldo, A. M., Barnett, J. L., Ber
- Page 122 and 123: Folkman, J. (2003). Fundamental con
- Page 124 and 125: Hofmann, S. M., Zhou, L., Perez-Til
- Page 126 and 127: Langin, D. (2006a). Adipose tissue
- Page 128 and 129: Ludwig, T., Ovitt, C. E., Bauer, U.
- Page 130 and 131: Nirde, P., Derocq, D., Maynadier, M
- Page 132 and 133: Rozanov, D. V., Hahn-Dantona, E., S
- Page 134 and 135: Taleb, S., Cancello, R., Clement, K
- Page 136 and 137: Xiao, Y., Junfeng, H., Tianhong, L.
- Page 138 and 139: F. ANNEXE98
- Page 140 and 141: Cathepsin D is a new ligand for ext
- Page 142 and 143: INTRODUCTIONLysosomal aspartic prot
- Page 144 and 145: pcDNA3.1(+)Myc-tagged LRP1b into pc
- Page 146 and 147: (Protein refolding kit, Novagen) fo
- Page 148 and 149:
anti-mouse-gold (Aurion). Sections
- Page 150 and 151:
not secrete detectable levels of pr
- Page 152 and 153:
using cath-D-/- MEF transfected wit
- Page 154 and 155:
co-culture outgrowth assays with ca
- Page 156 and 157:
REFERENCES1. Vignon, F., Capony, F.
- Page 158 and 159:
steps in vivo: proliferation, angio
- Page 160 and 161:
28. Laurent-Matha, V., Lucas, A., H
- Page 162 and 163:
42. Zurhove, K., Nakajima, C., Herz
- Page 164 and 165:
Figure 1. Cath-D interacts with the
- Page 166 and 167:
Figure 2. Cath-D binds to residues
- Page 168 and 169:
Figure 3. Cath-D interacts with LRP
- Page 170 and 171:
Figure 5. Silencing LRP1 in cath-D
- Page 172 and 173:
Figure 1. Cath-D interacts with the
- Page 174 and 175:
Figure 6. LRP1 is the receptor medi
- Page 176 and 177:
Beaujouin, Figure Sup. 2cath-D-/-ME
- Page 178:
RésuméLaspartyl protéase catheps