1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Discussion and Results 3<br />
planarity in a fashion that brings the carbon atom to be attacked in closer proximity to the<br />
reactive site.<br />
To get further insight, it was decided to repeat the ring closure with diamagnetic nickel(II) as<br />
central metal and to compute possible intermediate structure focusing of their geometry<br />
and orbital shapes utilizing the αα-conformer as model.<br />
The practical approach resulted in the formation of the corresponding nickel(II) complexes<br />
Ni(II)-67 and Ni(II)-68 in comparable overall yield and also comparable product ratio. Thus,<br />
any effect of a paramagnetic metal center is negligible and can be excluded from<br />
considerations. The theoretical modeling delivered pictorial insights like the one being<br />
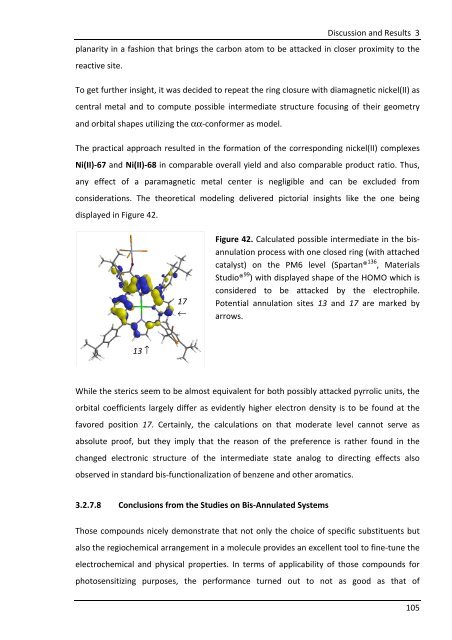
displayed in Figure 42.<br />
13 ↑<br />
17<br />
←<br />
Figure 42. Calculated possible intermediate in the bisannulation<br />
process with one closed ring (with attached<br />
catalyst) on the PM6 level (Spartan® 136 , Materials<br />
Studio® 99 ) with displayed shape of the HOMO which is<br />
considered to be attacked by the electrophile.<br />
Potential annulation sites 13 and 17 are marked by<br />
arrows.<br />
While the sterics seem to be almost equivalent for both possibly attacked pyrrolic units, the<br />
orbital coefficients largely differ as evidently higher electron density is to be found at the<br />
favored position 17. Certainly, the calculations on that moderate level cannot serve as<br />
absolute proof, but they imply that the reason of the preference is rather found in the<br />
changed electronic structure of the intermediate state analog to directing effects also<br />
observed in standard bis-functionalization of benzene and other aromatics.<br />
3.2.7.8 Conclusions from the Studies on Bis-Annulated Systems<br />
Those compounds nicely demonstrate that not only the choice of specific substituents but<br />
also the regiochemical arrangement in a molecule provides an excellent tool to fine-tune the<br />
electrochemical and physical properties. In terms of applicability of those compounds for<br />
photosensitizing purposes, the performance turned out to not as good as that of<br />
105