1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
6 Experimental Section<br />
13 C NMR (100.5 MHz, rt, CDCl3): δ [ppm] = 1<strong>1.1</strong>, 11.9, 12.0, 17.3, 19.3, 23.1, 29.8, 30.8, 48.0,<br />
49.9, 51.6, 51.6, 93.0, 97.1, 104.0, 106.0, 122.5, 128.3, 129.2, 130.5, 131.6, 135.8, 136.1,<br />
136.2, 137.8, 141.6, 145.0, 149.0, 150.8, 155.2, 160.3, 171.4, 173.6, 196.3.<br />
MS (FAB+, NBA): m/z = 549 (100) [M] +· .<br />
IR (ATR): 𝜈� [cm -1 ] = 3395, 2957, 1739, 1708, 1618, 1553, 1498, 1436, 1347, 1260, 1160, 1044,<br />
984, 908, 796, 734, 671.<br />
UV/Vis (CH2Cl2): λ [nm] (ε [M -1 ·cm -1 ]) = 413 (73800), 508 (8400), 538 (7800), 610 (6600), 648<br />
(32900).<br />
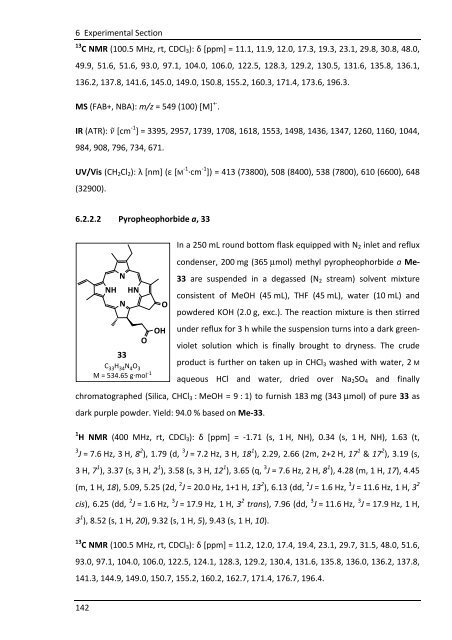
6.2.2.2 Pyropheophorbide a, 33<br />
142<br />
N<br />
NH HN<br />
N<br />
33<br />
O<br />
C 33 H 34 N 4 O 3<br />
M = 534.65 g·mol -1<br />
O<br />
OH<br />
In a 250 mL round bottom flask equipped with N2 inlet and reflux<br />
condenser, 200 mg (365 μmol) methyl pyropheophorbide a Me-<br />
33 are suspended in a degassed (N2 stream) solvent mixture<br />
consistent of MeOH (45 mL), THF (45 mL), water (10 mL) and<br />
powdered KOH (2.0 g, exc.). The reaction mixture is then stirred<br />
under reflux for 3 h while the suspension turns into a dark green-<br />
violet solution which is finally brought to dryness. The crude<br />
product is further on taken up in CHCl3 washed with water, 2 M<br />
aqueous HCl and water, dried over Na2SO4 and finally<br />
chromatographed (Silica, CHCl3 : MeOH = 9 : 1) to furnish 183 mg (343 μmol) of pure 33 as<br />
dark purple powder. Yield: 94.0 % based on Me-33.<br />
1 H NMR (400 MHz, rt, CDCl3): δ [ppm] = -1.71 (s, 1 H, NH), 0.34 (s, 1 H, NH), 1.63 (t,<br />
3 J = 7.6 Hz, 3 H, 8 2 ), 1.79 (d, 3 J = 7.2 Hz, 3 H, 18 1 ), 2.29, 2.66 (2m, 2+2 H, 17 1 & 17 2 ), 3.19 (s,<br />
3 H, 7 1 ), 3.37 (s, 3 H, 2 1 ), 3.58 (s, 3 H, 12 1 ), 3.65 (q, 3 J = 7.6 Hz, 2 H, 8 1 ), 4.28 (m, 1 H, 17), 4.45<br />
(m, 1 H, 18), 5.09, 5.25 (2d, 2 J = 20.0 Hz, 1+1 H, 13 2 ), 6.13 (dd, 2 J = 1.6 Hz, 3 J = 11.6 Hz, 1 H, 3 2<br />
cis), 6.25 (dd, 2 J = 1.6 Hz, 3 J = 17.9 Hz, 1 H, 3 2 trans), 7.96 (dd, 3 J = 11.6 Hz, 3 J = 17.9 Hz, 1 H,<br />
3 1 ), 8.52 (s, 1 H, 20), 9.32 (s, 1 H, 5), 9.43 (s, 1 H, 10).<br />
13 C NMR (100.5 MHz, rt, CDCl3): δ [ppm] = 11.2, 12.0, 17.4, 19.4, 23.1, 29.7, 31.5, 48.0, 51.6,<br />
93.0, 97.1, 104.0, 106.0, 122.5, 124.1, 128.3, 129.2, 130.4, 131.6, 135.8, 136.0, 136.2, 137.8,<br />
141.3, 144.9, 149.0, 150.7, 155.2, 160.2, 162.7, 171.4, 176.7, 196.4.