1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
6.2.2 Semi-Natural Cycloketo-Porphyrin Systems<br />
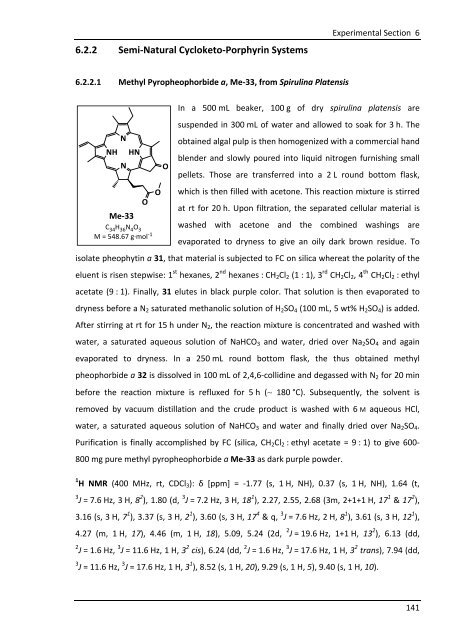
6.2.2.1 Methyl Pyropheophorbide a, Me-33, from Spirulina Platensis<br />
N<br />
NH HN<br />
N<br />
Me-33<br />
O<br />
C 34 H 36 N 4 O 3<br />
M = 548.67 g·mol -1<br />
O<br />
O<br />
Experimental Section 6<br />
In a 500 mL beaker, 100 g of dry spirulina platensis are<br />
suspended in 300 mL of water and allowed to soak for 3 h. The<br />
obtained algal pulp is then homogenized with a commercial hand<br />
blender and slowly poured into liquid nitrogen furnishing small<br />
pellets. Those are transferred into a 2 L round bottom flask,<br />
which is then filled with acetone. This reaction mixture is stirred<br />
at rt for 20 h. Upon filtration, the separated cellular material is<br />
washed with acetone and the combined washings are<br />
evaporated to dryness to give an oily dark brown residue. To<br />
isolate pheophytin a 31, that material is subjected to FC on silica whereat the polarity of the<br />
eluent is risen stepwise: 1 st hexanes, 2 nd hexanes : CH2Cl2 (1 : 1), 3 rd CH2Cl2, 4 th CH2Cl2 : ethyl<br />
acetate (9 : 1). Finally, 31 elutes in black purple color. That solution is then evaporated to<br />
dryness before a N2 saturated methanolic solution of H2SO4 (100 mL, 5 wt% H2SO4) is added.<br />
After stirring at rt for 15 h under N2, the reaction mixture is concentrated and washed with<br />
water, a saturated aqueous solution of NaHCO3 and water, dried over Na2SO4 and again<br />
evaporated to dryness. In a 250 mL round bottom flask, the thus obtained methyl<br />
pheophorbide a 32 is dissolved in 100 mL of 2,4,6-collidine and degassed with N2 for 20 min<br />
before the reaction mixture is refluxed for 5 h (∼ 180 °C). Subsequently, the solvent is<br />
removed by vacuum distillation and the crude product is washed with 6 M aqueous HCl,<br />
water, a saturated aqueous solution of NaHCO3 and water and finally dried over Na2SO4.<br />
Purification is finally accomplished by FC (silica, CH2Cl2 : ethyl acetate = 9 : 1) to give 600-<br />
800 mg pure methyl pyropheophorbide a Me-33 as dark purple powder.<br />
1 H NMR (400 MHz, rt, CDCl3): δ [ppm] = -1.77 (s, 1 H, NH), 0.37 (s, 1 H, NH), 1.64 (t,<br />
3 J = 7.6 Hz, 3 H, 8 2 ), 1.80 (d, 3 J = 7.2 Hz, 3 H, 18 1 ), 2.27, 2.55, 2.68 (3m, 2+1+1 H, 17 1 & 17 2 ),<br />
3.16 (s, 3 H, 7 1 ), 3.37 (s, 3 H, 2 1 ), 3.60 (s, 3 H, 17 4 & q, 3 J = 7.6 Hz, 2 H, 8 1 ), 3.61 (s, 3 H, 12 1 ),<br />
4.27 (m, 1 H, 17), 4.46 (m, 1 H, 18), 5.09, 5.24 (2d, 2 J = 19.6 Hz, 1+1 H, 13 2 ), 6.13 (dd,<br />
2 J = 1.6 Hz, 3 J = 11.6 Hz, 1 H, 3 2 cis), 6.24 (dd, 2 J = 1.6 Hz, 3 J = 17.6 Hz, 1 H, 3 2 trans), 7.94 (dd,<br />
3 J = 11.6 Hz, 3 J = 17.6 Hz, 1 H, 3 1 ), 8.52 (s, 1 H, 20), 9.29 (s, 1 H, 5), 9.40 (s, 1 H, 10).<br />
141