1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2 State of The Art & Aims<br />
They were developed by N. JUX and co-workers and proved excellently suited for the<br />
construction of several tailored systems with diverse applications. For example, highly<br />
charged porphyrins and their metal complexes were synthesized 70 for the investigation of<br />
fundamental biochemical subjects like the binding of NO to heme proteins under<br />
physiological conditions 71 or the modeling of cytochrome P450NOR 72 . Porphyrin crown ether<br />
conjugates were investigated as ditopic receptors 73 and metal complexes of inherently chiral<br />
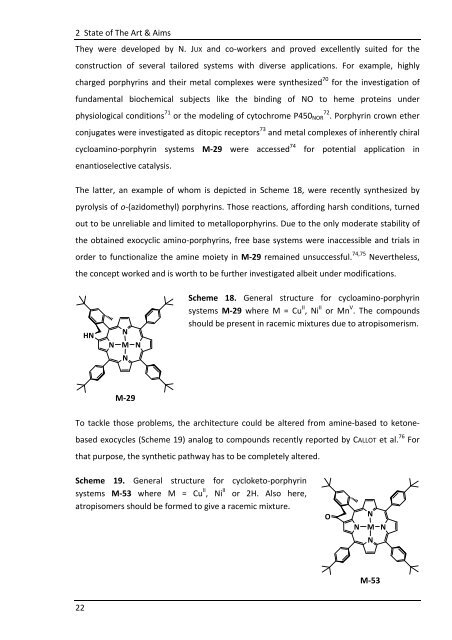
cycloamino-porphyrin systems M-29 were accessed 74 for potential application in<br />
enantioselective catalysis.<br />
The latter, an example of whom is depicted in Scheme 18, were recently synthesized by<br />
pyrolysis of o-(azidomethyl) porphyrins. Those reactions, affording harsh conditions, turned<br />
out to be unreliable and limited to metalloporphyrins. Due to the only moderate stability of<br />
the obtained exocyclic amino-porphyrins, free base systems were inaccessible and trials in<br />
order to functionalize the amine moiety in M-29 remained unsuccessful. 74,75 Nevertheless,<br />
the concept worked and is worth to be further investigated albeit under modifications.<br />
22<br />
HN<br />
N<br />
N<br />
M<br />
N<br />
M-29<br />
N<br />
Scheme 18. General structure for cycloamino-porphyrin<br />
systems M-29 where M = Cu II , Ni II or Mn V . The compounds<br />
should be present in racemic mixtures due to atropisomerism.<br />
To tackle those problems, the architecture could be altered from amine-based to ketone-<br />
based exocycles (Scheme 19) analog to compounds recently reported by CALLOT et al. 76 For<br />
that purpose, the synthetic pathway has to be completely altered.<br />
Scheme 19. General structure for cycloketo-porphyrin<br />
systems M-53 where M = Cu II , Ni II or 2H. Also here,<br />
atropisomers should be formed to give a racemic mixture.<br />
O<br />
N<br />
N<br />
M<br />
N<br />
M-53<br />
N