1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3 Discussion and Results<br />
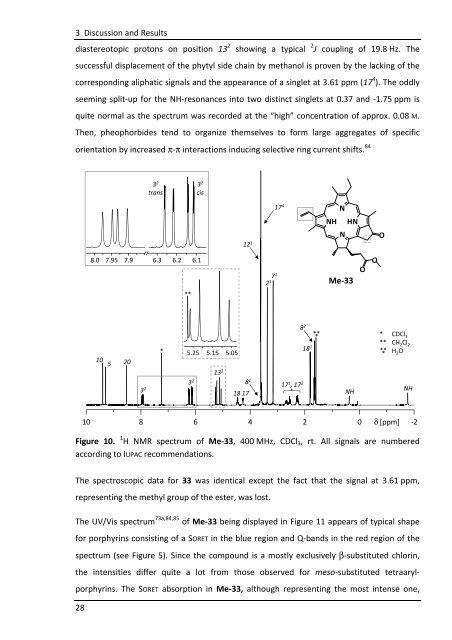
diastereotopic protons on position 13 2 showing a typical 2 J coupling of 19.8 Hz. The<br />
successful displacement of the phytyl side chain by methanol is proven by the lacking of the<br />
corresponding aliphatic signals and the appearance of a singlet at 3.61 ppm (17 4 ). The oddly<br />
seeming split-up for the NH-resonances into two distinct singlets at 0.37 and -1.75 ppm is<br />
quite normal as the spectrum was recorded at the “high” concentration of approx. 0.08 M.<br />
Then, pheophorbides tend to organize themselves to form large aggregates of specific<br />
orientation by increased π-π interactions inducing selective ring current shifts. 84<br />
28<br />
8.0 7.95 7.9 6.3 6.2 6.1<br />
10<br />
5 20<br />
3 1<br />
3 2<br />
trans<br />
*<br />
**<br />
5.25 5.15 5.05<br />
3 2<br />
3 2<br />
cis<br />
13 2<br />
12 1<br />
8 1<br />
18 17<br />
N<br />
NH HN<br />
* CDCl3 ** CH2Cl2 **<br />
* H2O 10 8 6 4 2 0 δ [ppm] -2<br />
21 71 17 4<br />
8 2<br />
17 1 , 17 2<br />
Figure 10. 1 H NMR spectrum of Me-33, 400 MHz, CDCl3, rt. All signals are numbered<br />
according to IUPAC recommendations.<br />
The spectroscopic data for 33 was identical except the fact that the signal at 3.61 ppm,<br />
representing the methyl group of the ester, was lost.<br />
18 1<br />
**<br />
*<br />
N<br />
Me-33<br />
The UV/Vis spectrum 73a,84,85 of Me-33 being displayed in Figure 11 appears of typical shape<br />
for porphyrins consisting of a SORET in the blue region and Q-bands in the red region of the<br />
spectrum (see Figure 5). Since the compound is a mostly exclusively β-substituted chlorin,<br />
the intensities differ quite a lot from those observed for meso-substituted tetraaryl-<br />
porphyrins. The SORET absorption in Me-33, although representing the most intense one,<br />
NH<br />
O<br />
O<br />
O<br />
NH