1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
N<br />
NH HN<br />
N<br />
MeO 2 C<br />
N<br />
NH HN<br />
N<br />
HO 2 C<br />
Discussion and Results 3<br />
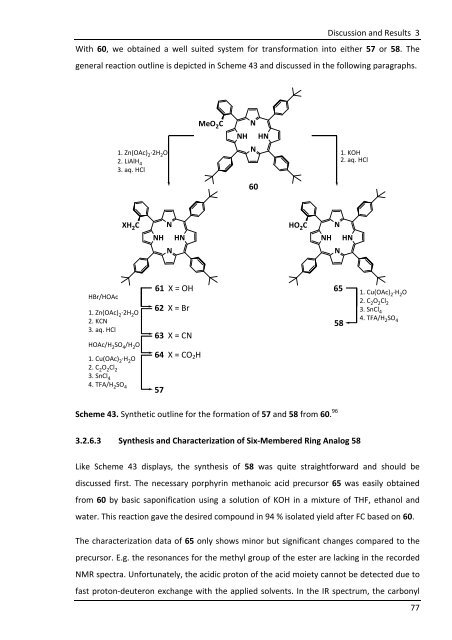
With 60, we obtained a well suited system for transformation into either 57 or 58. The<br />
general reaction outline is depicted in Scheme 43 and discussed in the following paragraphs.<br />
HBr/HOAc<br />
1. Zn(OAc) 2 ·2H 2 O<br />
2. LiAlH 4<br />
3. aq. HCl<br />
XH 2 C<br />
1. Zn(OAc) 2 ·2H 2 O<br />
2. KCN<br />
3. aq. HCl<br />
HOAc/H 2 SO 4 /H 2 O<br />
1. Cu(OAc) 2 ·H 2 O<br />
2. C 2 O 2 Cl 2<br />
3. SnCl 4<br />
4. TFA/H 2 SO 4<br />
61 X = OH<br />
62 X = Br<br />
63 X = CN<br />
64 X = CO2H<br />
57<br />
1. KOH<br />
2. aq. HCl<br />
N<br />
NH HN<br />
N<br />
Scheme 43. Synthetic outline for the formation of 57 and 58 from 60. 96<br />
3.2.6.3 Synthesis and Characterization of Six-Membered Ring Analog 58<br />
1. Cu(OAc) 2 ·H 2 O<br />
2. C 2 O 2 Cl 2<br />
3. SnCl 4<br />
4. TFA/H 2 SO 4<br />
Like Scheme 43 displays, the synthesis of 58 was quite straightforward and should be<br />
discussed first. The necessary porphyrin methanoic acid precursor 65 was easily obtained<br />
from 60 by basic saponification using a solution of KOH in a mixture of THF, ethanol and<br />
water. This reaction gave the desired compound in 94 % isolated yield after FC based on 60.<br />
The characterization data of 65 only shows minor but significant changes compared to the<br />
precursor. E.g. the resonances for the methyl group of the ester are lacking in the recorded<br />
NMR spectra. Unfortunately, the acidic proton of the acid moiety cannot be detected due to<br />
fast proton-deuteron exchange with the applied solvents. In the IR spectrum, the carbonyl<br />
60<br />
65<br />
58<br />
77