1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
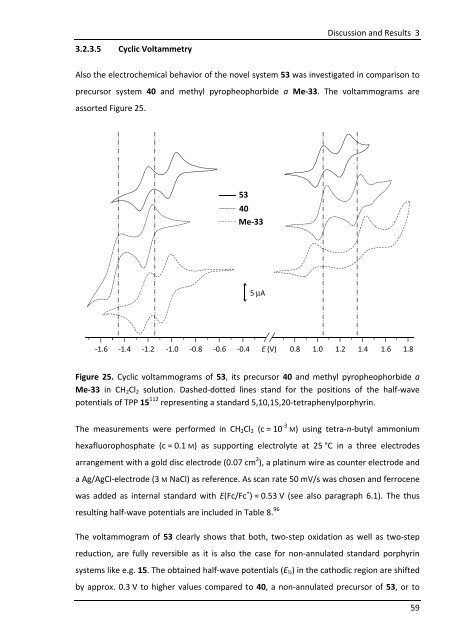
3.2.3.5 Cyclic Voltammetry<br />
-1.6<br />
5 μA<br />
Discussion and Results 3<br />
Also the electrochemical behavior of the novel system 53 was investigated in comparison to<br />
precursor system 40 and methyl pyropheophorbide a Me-33. The voltammograms are<br />
assorted Figure 25.<br />
53<br />
40<br />
Me-33<br />
-1.4 -1.2 -1.0 -0.8 -0.6 -0.4 E (V) 0.8 1.0 1.2 1.4 1.6 1.8<br />
Figure 25. Cyclic voltammograms of 53, its precursor 40 and methyl pyropheophorbide a<br />
Me-33 in CH2Cl2 solution. Dashed-dotted lines stand for the positions of the half-wave<br />
potentials of TPP 15 112 representing a standard 5,10,15,20-tetraphenylporphyrin.<br />
The measurements were performed in CH2Cl2 (c = 10 -3 M) using tetra-n-butyl ammonium<br />
hexafluorophosphate (c = 0.1 M) as supporting electrolyte at 25 °C in a three electrodes<br />
arrangement with a gold disc electrode (0.07 cm 2 ), a platinum wire as counter electrode and<br />
a Ag/AgCl-electrode (3 M NaCl) as reference. As scan rate 50 mV/s was chosen and ferrocene<br />
was added as internal standard with E(Fc/Fc + ) = 0.53 V (see also paragraph 6.1). The thus<br />
resulting half-wave potentials are included in Table 8. 96<br />
The voltammogram of 53 clearly shows that both, two-step oxidation as well as two-step<br />
reduction, are fully reversible as it is also the case for non-annulated standard porphyrin<br />
systems like e.g. 15. The obtained half-wave potentials (E½) in the cathodic region are shifted<br />
by approx. 0.3 V to higher values compared to 40, a non-annulated precursor of 53, or to<br />
59