1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
3 Discussion and Results<br />
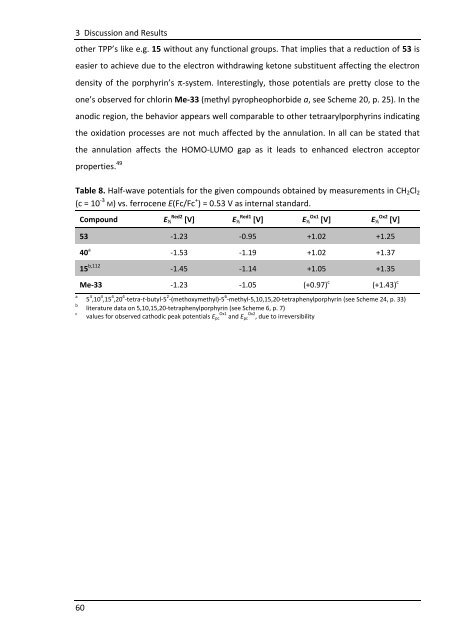
other TPP’s like e.g. 15 without any functional groups. That implies that a reduction of 53 is<br />
easier to achieve due to the electron withdrawing ketone substituent affecting the electron<br />
density of the porphyrin’s π-system. Interestingly, those potentials are pretty close to the<br />
one’s observed for chlorin Me-33 (methyl pyropheophorbide a, see Scheme 20, p. 25). In the<br />
anodic region, the behavior appears well comparable to other tetraarylporphyrins indicating<br />
the oxidation processes are not much affected by the annulation. In all can be stated that<br />
the annulation affects the HOMO-LUMO gap as it leads to enhanced electron acceptor<br />
properties. 49<br />
Table 8. Half-wave potentials for the given compounds obtained by measurements in CH2Cl2<br />
(c = 10 -3 M) vs. ferrocene E(Fc/Fc + ) = 0.53 V as internal standard.<br />
Compound E½ Red2 [V] E½ Red1 [V] E½ Ox1 [V] E½ Ox2 [V]<br />
53 -1.23 -0.95 +1.02 +1.25<br />
40 a<br />
15 b,112<br />
60<br />
-1.53 -<strong>1.1</strong>9 +1.02 +1.37<br />
-1.45 -<strong>1.1</strong>4 +1.05 +1.35<br />
Me-33 -1.23 -1.05 (+0.97) c<br />
(+1.43) c<br />
a 4 4 4 4 2 6<br />
5 ,10 ,15 ,20 -tetra-t-butyl-5 -(methoxymethyl)-5 -methyl-5,10,15,20-tetraphenylporphyrin (see Scheme 24, p. 33) .<br />
b<br />
literature data on 5,10,15,20-tetraphenylporphyrin (see Scheme 6, p. 7)<br />
c Ox1 Ox2<br />
values for observed cathodic peak potentials Epc and Epc , due to irreversibility