1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
3 Discussion and Results<br />
nitro compound 91, the signals are expectedly detected more upfield at 8.66 and 8.57 ppm<br />
(ortho-H) and at 8.11 and 8.33 ppm (meta-H) while they are observed at 8.33 and 8.15 ppm<br />
(ortho-H) and at 7.03 ppm (meta-H) in amino compound 92, respectively, due to the<br />
switching from a strongly electron withdrawing to a strongly electron donating functionality.<br />
The remainder resonances for the non-annulated phenyl ring, the tethered phenyl ring, the<br />
tether itself and the t-butyl groups appear unaffected and are found in nearly exactly the<br />
same spectral position as they are observed in non-functionalized 53. While the signal for<br />
the inner ring amine protons in 92, being found at -1.61 ppm, i.e. comparable to that in 53,<br />
the resonance is shifted upfield to -1.69 ppm in 91 again reflecting the impact of the<br />
different functionalities on the substituent in 15 position. Further NMR-data is included in<br />
the experimental section.<br />
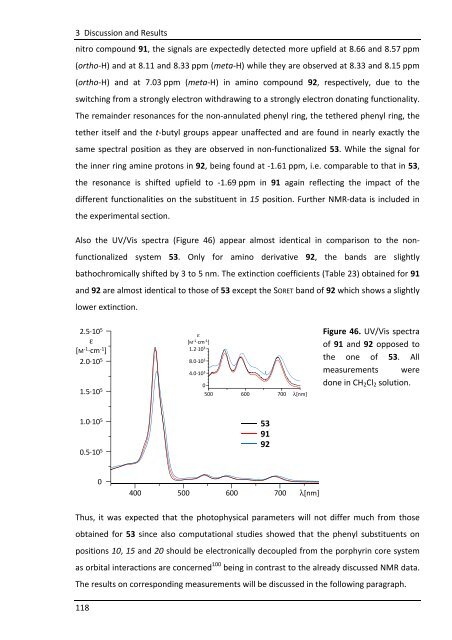
Also the UV/Vis spectra (Figure 46) appear almost identical in comparison to the non-<br />
functionalized system 53. Only for amino derivative 92, the bands are slightly<br />
bathochromically shifted by 3 to 5 nm. The extinction coefficients (Table 23) obtained for 91<br />
and 92 are almost identical to those of 53 except the SORET band of 92 which shows a slightly<br />
lower extinction.<br />
2.5·10 5<br />
ε<br />
[M -1 ·cm -1 ]<br />
2.0·10 5<br />
1.5·10 5<br />
1.0·10 5<br />
0.5·10 5<br />
118<br />
0<br />
ε<br />
[M -1 ·cm -1 ]<br />
1.2·10 3<br />
8.0·10 3<br />
4.0·10 3<br />
0<br />
500 600 700<br />
400 500 600 700<br />
λ[nm]<br />
λ[nm]<br />
Figure 46. UV/Vis spectra<br />
of 91 and 92 opposed to<br />
the one of 53. All<br />
measurements were<br />
done in CH2Cl2 solution.<br />
Thus, it was expected that the photophysical parameters will not differ much from those<br />
obtained for 53 since also computational studies showed that the phenyl substituents on<br />
positions 10, 15 and 20 should be electronically decoupled from the porphyrin core system<br />
as orbital interactions are concerned 100 being in contrast to the already discussed NMR data.<br />
The results on corresponding measurements will be discussed in the following paragraph.<br />
53<br />
91<br />
92