1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
3 Discussion and Results<br />
With all this in mind, a nitro group (-NO2) seemed the subject of choice since it perfectly fit<br />
with the abovementioned items and as 4-nitrobenzaldehyde is commercially available.<br />
Hence, the first step was to synthesize the appropriate AB2C-type porphyrin system and then<br />
convert it into the corresponding nitro-phenylporphyrin carboxylic acid.<br />
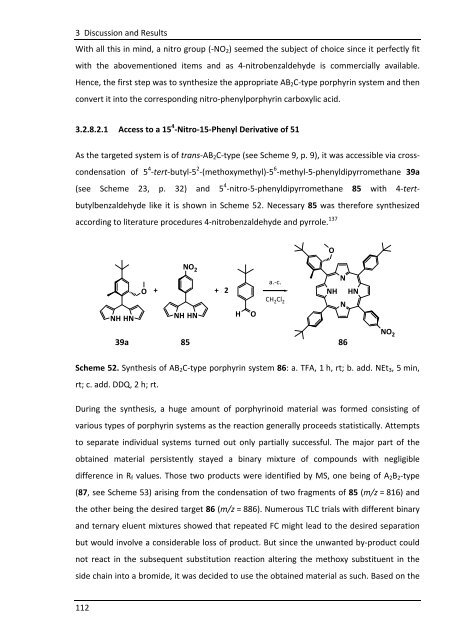
3.2.8.2.1 Access to a 15 4 -Nitro-15-Phenyl Derivative of 51<br />
As the targeted system is of trans-AB2C-type (see Scheme 9, p. 9), it was accessible via cross-<br />
condensation of 5 4 -tert-butyl-5 2 -(methoxymethyl)-5 6 -methyl-5-phenyldipyrromethane 39a<br />
(see Scheme 23, p. 32) and 5 4 -nitro-5-phenyldipyrromethane 85 with 4-tert-<br />
butylbenzaldehyde like it is shown in Scheme 52. Necessary 85 was therefore synthesized<br />
according to literature procedures 4-nitrobenzaldehyde and pyrrole. 137<br />
112<br />
NH HN<br />
O<br />
+<br />
NO 2<br />
NH HN<br />
+ 2<br />
H<br />
O<br />
a.-c.<br />
CH 2 Cl 2<br />
O<br />
N<br />
NH HN<br />
39a 85 86<br />
Scheme 52. Synthesis of AB2C-type porphyrin system 86: a. TFA, 1 h, rt; b. add. NEt3, 5 min,<br />
rt; c. add. DDQ, 2 h; rt.<br />
During the synthesis, a huge amount of porphyrinoid material was formed consisting of<br />
various types of porphyrin systems as the reaction generally proceeds statistically. Attempts<br />
to separate individual systems turned out only partially successful. The major part of the<br />
obtained material persistently stayed a binary mixture of compounds with negligible<br />
difference in Rf values. Those two products were identified by MS, one being of A2B2-type<br />
(87, see Scheme 53) arising from the condensation of two fragments of 85 (m/z = 816) and<br />
the other being the desired target 86 (m/z = 886). Numerous TLC trials with different binary<br />
and ternary eluent mixtures showed that repeated FC might lead to the desired separation<br />
but would involve a considerable loss of product. But since the unwanted by-product could<br />
not react in the subsequent substitution reaction altering the methoxy substituent in the<br />
side chain into a bromide, it was decided to use the obtained material as such. Based on the<br />
N<br />
NO 2