1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3 Discussion and Results<br />
peak at m/z = 1369 correspondent to [M] + in the MALDI-TOF spectrum without any<br />
fragments.<br />
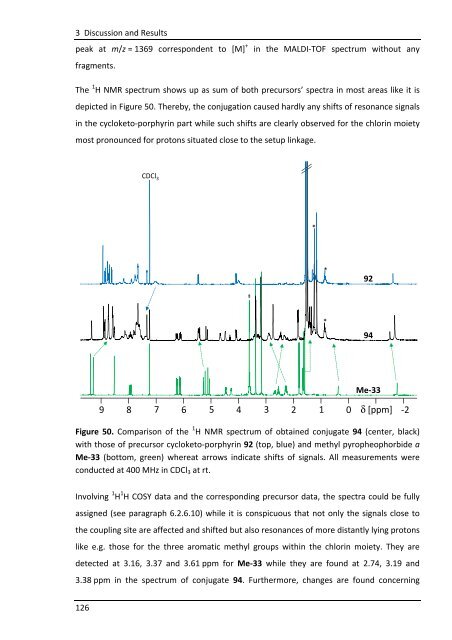
The 1 H NMR spectrum shows up as sum of both precursors’ spectra in most areas like it is<br />
depicted in Figure 50. Thereby, the conjugation caused hardly any shifts of resonance signals<br />
in the cycloketo-porphyrin part while such shifts are clearly observed for the chlorin moiety<br />
most pronounced for protons situated close to the setup linkage.<br />
126<br />
CDCl 3<br />
9 8 7 6 5 4 3 2 1 0 δ [ppm] -2<br />
Figure 50. Comparison of the 1 H NMR spectrum of obtained conjugate 94 (center, black)<br />
with those of precursor cycloketo-porphyrin 92 (top, blue) and methyl pyropheophorbide a<br />
Me-33 (bottom, green) whereat arrows indicate shifts of signals. All measurements were<br />
conducted at 400 MHz in CDCl3 at rt.<br />
Involving 1 H 1 H COSY data and the corresponding precursor data, the spectra could be fully<br />
assigned (see paragraph 6.2.6.10) while it is conspicuous that not only the signals close to<br />
the coupling site are affected and shifted but also resonances of more distantly lying protons<br />
like e.g. those for the three aromatic methyl groups within the chlorin moiety. They are<br />
detected at 3.16, 3.37 and 3.61 ppm for Me-33 while they are found at 2.74, 3.19 and<br />
3.38 ppm in the spectrum of conjugate 94. Furthermore, changes are found concerning<br />
‡<br />
*<br />
*<br />
*<br />
*<br />
92<br />
94<br />
Me-33