1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3 Discussion and Results<br />
3.2.2.2 FRIEDEL-CRAFTS Acylation to Form the Keto-Exocycle<br />
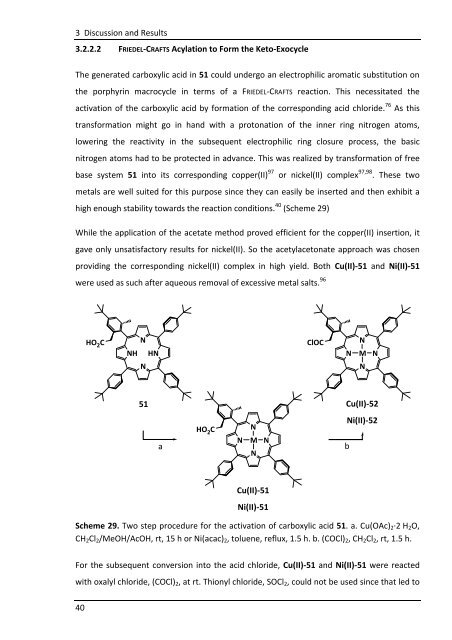
The generated carboxylic acid in 51 could undergo an electrophilic aromatic substitution on<br />
the porphyrin macrocycle in terms of a FRIEDEL-CRAFTS reaction. This necessitated the<br />
activation of the carboxylic acid by formation of the corresponding acid chloride. 76 As this<br />
transformation might go in hand with a protonation of the inner ring nitrogen atoms,<br />
lowering the reactivity in the subsequent electrophilic ring closure process, the basic<br />
nitrogen atoms had to be protected in advance. This was realized by transformation of free<br />
base system 51 into its corresponding copper(II) 97 or nickel(II) complex 97,98 . These two<br />
metals are well suited for this purpose since they can easily be inserted and then exhibit a<br />
high enough stability towards the reaction conditions. 40 (Scheme 29)<br />
While the application of the acetate method proved efficient for the copper(II) insertion, it<br />
gave only unsatisfactory results for nickel(II). So the acetylacetonate approach was chosen<br />
providing the corresponding nickel(II) complex in high yield. Both Cu(II)-51 and Ni(II)-51<br />
were used as such after aqueous removal of excessive metal salts. 96<br />
40<br />
HO 2 C<br />
N<br />
NH HN<br />
N<br />
51<br />
ClOC<br />
HO2C N<br />
Ni(II)-52<br />
a<br />
N M<br />
N<br />
N<br />
b<br />
Cu(II)-51<br />
Ni(II)-51<br />
Scheme 29. Two step procedure for the activation of carboxylic acid 51. a. Cu(OAc)2·2 H2O,<br />
CH2Cl2/MeOH/AcOH, rt, 15 h or Ni(acac)2, toluene, reflux, 1.5 h. b. (COCl)2, CH2Cl2, rt, 1.5 h.<br />
For the subsequent conversion into the acid chloride, Cu(II)-51 and Ni(II)-51 were reacted<br />
with oxalyl chloride, (COCl)2, at rt. Thionyl chloride, SOCl2, could not be used since that led to<br />
N<br />
N<br />
M<br />
N<br />
N<br />
Cu(II)-52 52