1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
3 Discussion and Results<br />
3.2.6.5 Comparative Studies<br />
Since the characterization data of cycloketo-porphyrin systems 53, 57 and 58 is very closely<br />
related to each other, they should be compared concerning not only basic characterization<br />
data but also in terms of their photophysical and electrochemical properties. Before we go<br />
into that matter, it shall again be started with theoretical aspects and visualizations based on<br />
computational studies.<br />
3.2.6.5.1 Computer-Assisted Simulations<br />
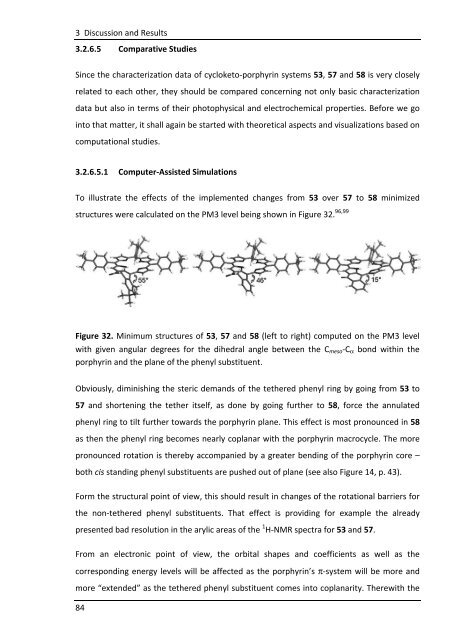
To illustrate the effects of the implemented changes from 53 over 57 to 58 minimized<br />
structures were calculated on the PM3 level being shown in Figure 32. 96,99<br />
Figure 32. Minimum structures of 53, 57 and 58 (left to right) computed on the PM3 level<br />
with given angular degrees for the dihedral angle between the Cmeso-Cα bond within the<br />
porphyrin and the plane of the phenyl substituent.<br />
Obviously, diminishing the steric demands of the tethered phenyl ring by going from 53 to<br />
57 and shortening the tether itself, as done by going further to 58, force the annulated<br />
phenyl ring to tilt further towards the porphyrin plane. This effect is most pronounced in 58<br />
as then the phenyl ring becomes nearly coplanar with the porphyrin macrocycle. The more<br />
pronounced rotation is thereby accompanied by a greater bending of the porphyrin core –<br />
both cis standing phenyl substituents are pushed out of plane (see also Figure 14, p. 43).<br />
Form the structural point of view, this should result in changes of the rotational barriers for<br />
the non-tethered phenyl substituents. That effect is providing for example the already<br />
presented bad resolution in the arylic areas of the 1 H-NMR spectra for 53 and 57.<br />
From an electronic point of view, the orbital shapes and coefficients as well as the<br />
corresponding energy levels will be affected as the porphyrin’s π-system will be more and<br />
more “extended” as the tethered phenyl substituent comes into coplanarity. Therewith the<br />
84