1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
O<br />
N<br />
NH HN<br />
N<br />
O<br />
N<br />
NH HN<br />
N<br />
Discussion and Results 3<br />
3.2.8 Cycloketo-Porphyrin Systems with Additional Functionality<br />
To exploit the properties of the synthesized cycloketo-porphyrin systems for applications in<br />
terms of PDT or any other technical device, it was necessary to modify the structure of 53 to<br />
gain the ability of attaching it covalently to antibodies, multiplying units or functional<br />
surfaces. 68<br />
Based on the aforementioned findings, it was decided to leave the exocycle as well as the<br />
remaining β-positions untouched since the electrochemical and photophysical behavior<br />
might else be negatively affected. Also the oppositely lying methyl substituent should not be<br />
changed as an enlargement would raise the steric hindrance hampering the closure of the<br />
exocyclic ring. So modifications are to be carried out on one of the three peripheral t-butyl<br />
phenyl substituents.<br />
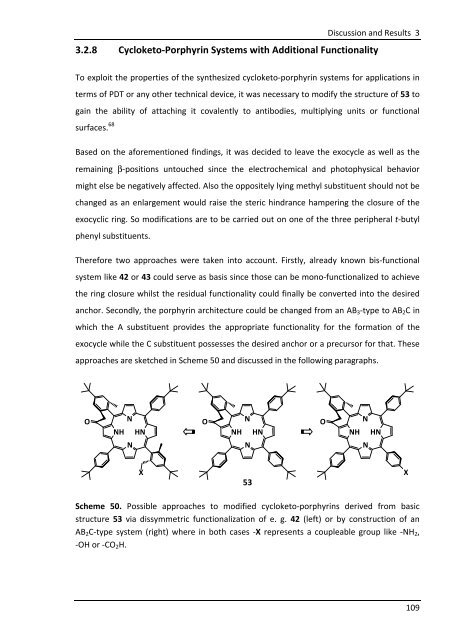
Therefore two approaches were taken into account. Firstly, already known bis-functional<br />
system like 42 or 43 could serve as basis since those can be mono-functionalized to achieve<br />
the ring closure whilst the residual functionality could finally be converted into the desired<br />
anchor. Secondly, the porphyrin architecture could be changed from an AB3-type to AB2C in<br />
which the A substituent provides the appropriate functionality for the formation of the<br />
exocycle while the C substituent possesses the desired anchor or a precursor for that. These<br />
approaches are sketched in Scheme 50 and discussed in the following paragraphs.<br />
X<br />
53<br />
O<br />
N<br />
NH HN<br />
N<br />
Scheme 50. Possible approaches to modified cycloketo-porphyrins derived from basic<br />
structure 53 via dissymmetric functionalization of e. g. 42 (left) or by construction of an<br />
AB2C-type system (right) where in both cases -X represents a coupleable group like -NH2,<br />
-OH or -CO2H.<br />
X<br />
109