1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
1.1 Porphyrins - Friedrich-Alexander-Universität Erlangen-Nürnberg
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3 Discussion and Results<br />
phenyl substituents. For 53 and 57 those are clearly lowered since a significant line<br />
broadening is observed representing the presence of a coalescence-like state. Connatural<br />
situations are also found in non-annulated tetraarylporphyrins with distinguishable half-<br />
spaces but at higher temperatures. As for 58, the exocyclic structure seems to be flexible<br />
itself, no definite statement is possible according to the underlying data.<br />
Since the rising tilt of the tethered phenyl ring is supposed to affect the electronic structure<br />
of the porphyrin core by mingling with its orbitals, variations in the porphyrin’s ring current<br />
are to be observed resulting in significant shifts of resonances for protons inside or situated<br />
directly on the periphery of the macrocycle. And indeed, this is detected for all studied<br />
compounds whereas the shifts seem to be the more pronounced the more the tethered<br />
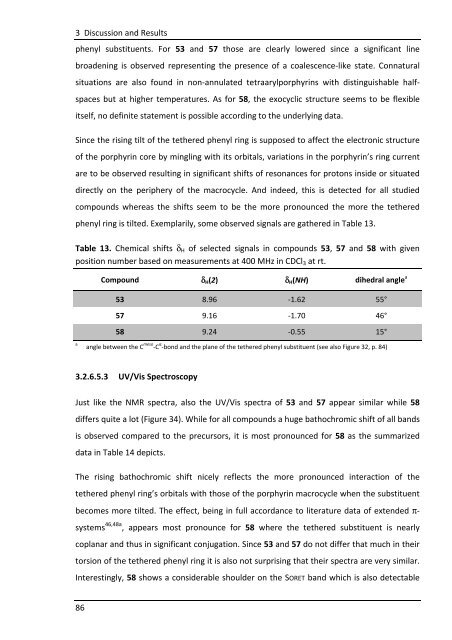
phenyl ring is tilted. Exemplarily, some observed signals are gathered in Table 13.<br />
Table 13. Chemical shifts δH of selected signals in compounds 53, 57 and 58 with given<br />
position number based on measurements at 400 MHz in CDCl3 at rt.<br />
a<br />
86<br />
Compound δH(2) δH(NH) dihedral angle a<br />
53 8.96 -1.62 55°<br />
57 9.16 -1.70 46°<br />
58 9.24 -0.55 15°<br />
angle between the C meso -C α -bond and the plane of the tethered phenyl substituent (see also Figure 32, p. 84) a<br />
3.2.6.5.3 UV/Vis Spectroscopy<br />
Just like the NMR spectra, also the UV/Vis spectra of 53 and 57 appear similar while 58<br />
differs quite a lot (Figure 34). While for all compounds a huge bathochromic shift of all bands<br />
is observed compared to the precursors, it is most pronounced for 58 as the summarized<br />
data in Table 14 depicts.<br />
The rising bathochromic shift nicely reflects the more pronounced interaction of the<br />
tethered phenyl ring’s orbitals with those of the porphyrin macrocycle when the substituent<br />
becomes more tilted. The effect, being in full accordance to literature data of extended π-<br />
systems 46,48a , appears most pronounce for 58 where the tethered substituent is nearly<br />
coplanar and thus in significant conjugation. Since 53 and 57 do not differ that much in their<br />
torsion of the tethered phenyl ring it is also not surprising that their spectra are very similar.<br />
Interestingly, 58 shows a considerable shoulder on the SORET band which is also detectable