Optimalisatie van de werkingsprocessen van het Bijzonder ... - KCE
Optimalisatie van de werkingsprocessen van het Bijzonder ... - KCE
Optimalisatie van de werkingsprocessen van het Bijzonder ... - KCE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
202 Special Solidarity Fund <strong>KCE</strong> Reports 133<br />
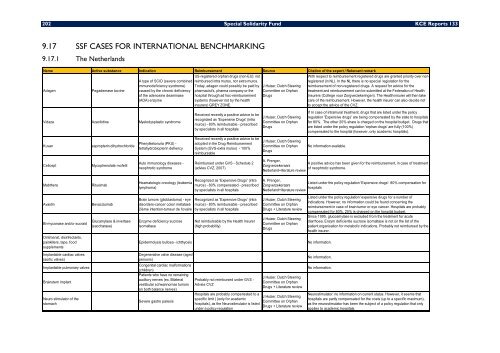
9.17 SSF CASES FOR INTERNATIONAL BENCHMARKING<br />
9.17.1 The Netherlands<br />
Name Active substance Indication Reimbursement Source Citation of the expert / Reke<strong>van</strong>t remark<br />
Adagen Pega<strong>de</strong>mase bovine<br />
A type of SCID (severe combined<br />
immuno<strong>de</strong>ficiency syndrome)<br />
caused by the chronic <strong>de</strong>ficiency<br />
of the a<strong>de</strong>nosine <strong>de</strong>aminase<br />
(ADA) enzyme<br />
Vidaza Azacitidine Myelodysplastic syndrome<br />
Ku<strong>van</strong> sapropterin dihydrochlori<strong>de</strong><br />
Cellcept Mycophenolate mofetil<br />
Mabthera Rituximab<br />
Avastin Bevacizumab<br />
Bi-myconase and/or sucraid<br />
Ointmenst, disinfectants,<br />
painkillers, tape, food<br />
supplements<br />
Implantable cardiac valves<br />
(aortic valves)<br />
Implantable pulmonary valves<br />
Brainstem implant<br />
Neuro stimulator of the<br />
stomach<br />
Glucamylase & invertase<br />
(saccharase)<br />
Phenylketonuria (PKU) -<br />
tetrahydrobiopterin <strong>de</strong>fiency<br />
Auto immunology diseases -<br />
neophrotic syndrome<br />
US-registered orphan drugs (non-EU): not<br />
reimbursed intra muros, nor extra muros.<br />
Today, adagen could possibly be paid by<br />
pharmacist's, pharma company or the<br />
hospital through ad hoc-reimbursement<br />
systems (however not by the health<br />
insurers) GREY ZONE.<br />
Received recently a positive advice to be<br />
recognized as 'Expensive Drugs' (intra<br />
muros) - 80% reimbursable - prescribed<br />
by specialists in all hospitals<br />
Received recently a positive advice to be<br />
adopted in the Drug Reimbursement<br />
System (GVS-extra muros) - 100%<br />
reimbursable<br />
Reimbursed un<strong>de</strong>r GVS - Schedule 2<br />
(advies CVZ, 2007)<br />
Recognized as 'Expensive Drugs' (intra<br />
Heamatologic oncology (leukemia -<br />
muros) - 80% compensated - prescribed<br />
lymphoma)<br />
by specialists in all hospitals<br />
Brain tumors (glioblastoma) - eye<br />
disor<strong>de</strong>rs-cancer colon métatasé<br />
2ième intention-tumeur <strong>de</strong> l'ovaire<br />
Enzyme <strong>de</strong>ficiency sucrose<br />
isomaltase<br />
Recognized as 'Expensive Drugs' (intra<br />
muros) - 80% reimbursable - prescribed<br />
by specialists in all hospitals<br />
Not reimbursable by the Health Insurer<br />
(high probability)<br />
J.Huizer, Dutch Steering<br />
Committee on Orphan<br />
Drugs<br />
J.Huizer, Dutch Steering<br />
Committee on Orphan<br />
Drugs<br />
J.Huizer, Dutch Steering<br />
Committee on Orphan<br />
Drugs<br />
With respect to reimbursement registered drugs are granted priority over nonregistered<br />
(in NL). In the NL there is no special legislation for the<br />
reimbursement of non-registered drugs. A request for advice for the<br />
treatment and reimbursement can be submitted at the Fe<strong>de</strong>ration of Health<br />
Insurers (College voor Zorgverzekeringen). The Health insurer will then take<br />
care of the reimbursement. However, the health insurer can also <strong>de</strong>ci<strong>de</strong> not<br />
to accept the advice of the CVZ.<br />
If In case of intramural treatment, drugs that are listed un<strong>de</strong>r the policy<br />
regulation 'Expensive drugs' are being compensated by the state to hospitals<br />
for 80%. The other 20% share is charged on the hospital budget. Drugs that<br />
are listed un<strong>de</strong>r the policy regulation 'orphan drugs' are fully (100%)<br />
compensated to the hospital (however, only aca<strong>de</strong>mic hospitals).<br />
No information available.<br />
A. Prenger,<br />
A positive advice has been given for the reimbursement, in case of treatment<br />
Zorgverzekeraars<br />
of neophrotic syndrome.<br />
Ne<strong>de</strong>rland+literature review<br />
A. Prenger,<br />
Listed un<strong>de</strong>r the policy regulation 'Expensive drugs': 80% compensation for<br />
Zorgverzekeraars<br />
hospitals<br />
Ne<strong>de</strong>rland+literature review<br />
J.Huizer, Dutch Steering<br />
Committee on Orphan<br />
Drugs + Literature review<br />
J.Huizer, Dutch Steering<br />
Committee on Orphan<br />
Drugs<br />
Epi<strong>de</strong>rmolysis bullosa - ichthyosis No information.<br />
Degenerative valve disease (aged<br />
persons)<br />
Congenital cardiac malformations<br />
(children)<br />
Patients who have no remaining<br />
auditory nerves (ex. Bilateral<br />
vestibular schwannomas tumors<br />
on both balance nerves)<br />
Severe gastro paresis<br />
Probably not reimbursed un<strong>de</strong>r GVS -<br />
Advies CVZ<br />
Hospitals are probably compensated to a<br />
specific limit ( (only for aca<strong>de</strong>mic<br />
hospitals), as the Neurostimulator is listed<br />
un<strong>de</strong>r a policy regulation<br />
J.Huizer, Dutch Steering<br />
Committee on Orphan<br />
Drugs + Literature review<br />
J.Huizer, Dutch Steering<br />
Committee on Orphan<br />
Drugs + Literature review<br />
Listed un<strong>de</strong>r the policy regulation 'expensive drugs for a number of<br />
indications. However, no information could be found concerning the<br />
reimbursement in case of brain tumor or eye cancer. Hospitals are probably<br />
compensated for 80%. 20% is charged on the hospital budget.<br />
Since 1995, glucoamylase is exclu<strong>de</strong>d from the treatment for acute<br />
diarrhoea. Enzym <strong>de</strong>ficientie sucrose isomaltase is not on the list of the<br />
patient organisation for metabolic indications. Probably not reimbursed by the<br />
health insurer.<br />
No information.<br />
No information.<br />
Neurostimulator: no information on current status. However, it seems that<br />
hospitals are partly compensated for the costs (up to a specific maximum),<br />
as the neurostimulator has been the subject of a policy regulation that only<br />
applies to aca<strong>de</strong>mic hospitals.