Experimental and Numerical Analysis of a PCM-Supported ...
Experimental and Numerical Analysis of a PCM-Supported ...
Experimental and Numerical Analysis of a PCM-Supported ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
The apparent heat capacity method is adopted in this study, since the temperature<br />
field is the primary dependent variable that can be derived directly from the solution.<br />
The apparent heat capacity assumes that the phase change occurs over a small<br />
temperature interval rather than at a sharp melting temperature. However, in the<br />
present study, all the three types <strong>of</strong> <strong>PCM</strong> packing media have a melting temperature<br />
interval <strong>of</strong> 2°C around the specified melting points according to the manufacturer’s<br />
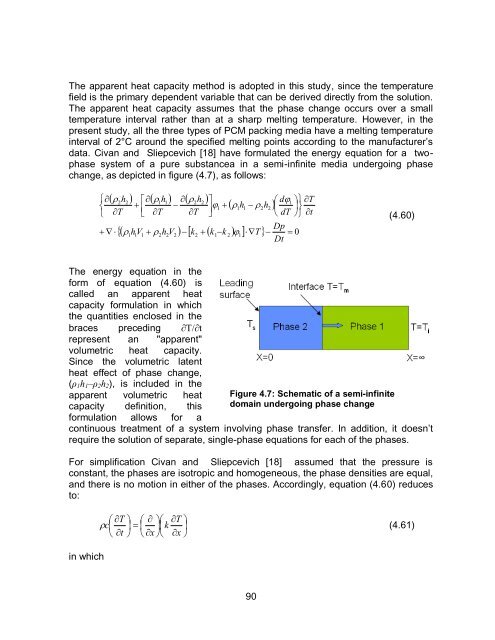
data. Civan <strong>and</strong> Sliepcevich [18] have formulated the energy equation for a twophase<br />
system <strong>of</strong> a pure substancea in a semi-infinite media undergoing phase<br />
change, as depicted in figure (4.7), as follows:<br />
<br />
<br />
h <br />
h <br />
h <br />
<br />
2<br />
<br />
T<br />
2<br />
hV h V <br />
k<br />
k<br />
k<br />
<br />
T<br />
0<br />
1<br />
<br />
<br />
<br />
<br />
1<br />
1<br />
1<br />
T<br />
2<br />
1<br />
2<br />
<br />
2<br />
2<br />
T<br />
2<br />
2<br />
<br />
1<br />
<br />
<br />
1<br />
<br />
2<br />
h h<br />
1<br />
1<br />
1<br />
2<br />
2<br />
d1<br />
<br />
T<br />
<br />
<br />
dT <br />
t<br />
Dp<br />
Dt<br />
(4.60)<br />
The energy equation in the<br />
form <strong>of</strong> equation (4.60) is<br />
called an apparent heat<br />
capacity formulation in which<br />
the quantities enclosed in the<br />
braces preceding T/t<br />
represent an "apparent"<br />
volumetric heat capacity.<br />
Since the volumetric latent<br />
heat effect <strong>of</strong> phase change,<br />
(ρ 1 h 1 –ρ 2 h 2 ), is included in the<br />
apparent volumetric heat<br />
capacity definition, this<br />
formulation allows for a<br />
continuous treatment <strong>of</strong> a system involving phase transfer. In addition, it doesn’t<br />
require the solution <strong>of</strong> separate, single-phase equations for each <strong>of</strong> the phases.<br />
For simplification Civan <strong>and</strong> Sliepcevich [18] assumed that the pressure is<br />
constant, the phases are isotropic <strong>and</strong> homogeneous, the phase densities are equal,<br />
<strong>and</strong> there is no motion in either <strong>of</strong> the phases. Accordingly, equation (4.60) reduces<br />
to:<br />
in which<br />
Figure 4.7: Schematic <strong>of</strong> a semi-infinite<br />
domain undergoing phase change<br />
T<br />
<br />
T<br />
<br />
c<br />
k<br />
<br />
(4.61)<br />
t<br />
x<br />
<br />
x<br />
<br />
90