Experimental and Numerical Analysis of a PCM-Supported ...
Experimental and Numerical Analysis of a PCM-Supported ...
Experimental and Numerical Analysis of a PCM-Supported ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
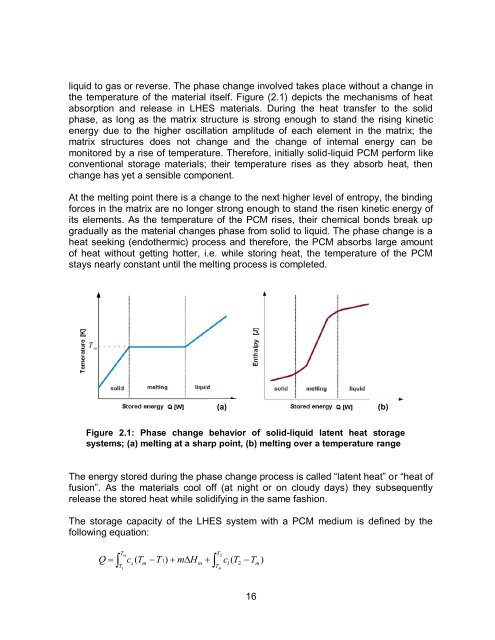
liquid to gas or reverse. The phase change involved takes place without a change in<br />
the temperature <strong>of</strong> the material itself. Figure (2.1) depicts the mechanisms <strong>of</strong> heat<br />
absorption <strong>and</strong> release in LHES materials. During the heat transfer to the solid<br />
phase, as long as the matrix structure is strong enough to st<strong>and</strong> the rising kinetic<br />
energy due to the higher oscillation amplitude <strong>of</strong> each element in the matrix; the<br />
matrix structures does not change <strong>and</strong> the change <strong>of</strong> internal energy can be<br />
monitored by a rise <strong>of</strong> temperature. Therefore, initially solid-liquid <strong>PCM</strong> perform like<br />
conventional storage materials; their temperature rises as they absorb heat, then<br />
change has yet a sensible component.<br />
At the melting point there is a change to the next higher level <strong>of</strong> entropy, the binding<br />
forces in the matrix are no longer strong enough to st<strong>and</strong> the risen kinetic energy <strong>of</strong><br />
its elements. As the temperature <strong>of</strong> the <strong>PCM</strong> rises, their chemical bonds break up<br />
gradually as the material changes phase from solid to liquid. The phase change is a<br />
heat seeking (endothermic) process <strong>and</strong> therefore, the <strong>PCM</strong> absorbs large amount<br />
<strong>of</strong> heat without getting hotter, i.e. while storing heat, the temperature <strong>of</strong> the <strong>PCM</strong><br />
stays nearly constant until the melting process is completed.<br />
(a)<br />
(b)<br />
Figure 2.1: Phase change behavior <strong>of</strong> solid-liquid latent heat storage<br />
systems; (a) melting at a sharp point, (b) melting over a temperature range<br />
The energy stored during the phase change process is called “latent heat” or “heat <strong>of</strong><br />
fusion”. As the materials cool <strong>of</strong>f (at night or on cloudy days) they subsequently<br />
release the stored heat while solidifying in the same fashion.<br />
The storage capacity <strong>of</strong> the LHES system with a <strong>PCM</strong> medium is defined by the<br />
following equation:<br />
Q <br />
<br />
T<br />
T<br />
1<br />
2<br />
cs( Tm<br />
T1)<br />
mH<br />
m<br />
cl<br />
( T2<br />
Tm<br />
)<br />
m<br />
<br />
T<br />
T<br />
m<br />
16