(GP/GT) for Additional Water Supply in the Lower Rio Grande
(GP/GT) for Additional Water Supply in the Lower Rio Grande
(GP/GT) for Additional Water Supply in the Lower Rio Grande
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
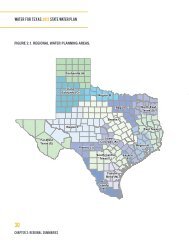
m-6<br />
A2: THE REVERSE OSMOSIS PROCESS<br />
The oldest of <strong>the</strong> membrane-related processes is <strong>the</strong> Reverse Osmosis (RO) Process, <strong>the</strong> earliest<br />
<strong>in</strong>stallations of which date back to be<strong>for</strong>e 1950, no matter that <strong>the</strong>se early units were strictly laboratorysized<br />
and often were more trouble than <strong>the</strong>y were worth. Shipboard-sized units, capable of desalt<strong>in</strong>g up<br />
to 15,000 gallons per day of seawater, appeared on vessels <strong>in</strong> <strong>the</strong> early Sixties. It was not until 1966 that<br />
commercial-sized units, capable of desalt<strong>in</strong>g up to 100,000 <strong>GP</strong>O, were <strong>in</strong>stalled <strong>in</strong> municipal use, one<br />
of <strong>the</strong>se early types be<strong>in</strong>g <strong>in</strong>stalled <strong>in</strong> <strong>the</strong> City of Pla<strong>in</strong>s, Texas, <strong>in</strong> 1967.<br />
The RO Process relies on a natural phenomenon: Osmosis, <strong>in</strong>volv<strong>in</strong>g fluid flow across a membrane called<br />
"semipermeable". The term arises from <strong>the</strong> fact that certa<strong>in</strong> components of a solution, usually <strong>the</strong><br />
solvent, can pass through such a membrane while o<strong>the</strong>rs, usually dissolved solids, cannot. The direction<br />
of solvent flow is determ<strong>in</strong>ed by its chemical potential which is a function of pressure, temperature and<br />
<strong>the</strong> concentration of <strong>the</strong> dissolved solids.<br />
Thus, if pure water is on both sides of a semipermeable membrane at equal pressure and temperature,<br />
no net flow can be realized <strong>in</strong>asmuch as <strong>the</strong> chemical potential is equal on both sides. If a soluble salt<br />
is now added to one side of <strong>the</strong> membrane, <strong>the</strong> potential on that side is reduced, caus<strong>in</strong>g flow to occur<br />
from <strong>the</strong> pure water side to <strong>the</strong> salt water side, thus dilut<strong>in</strong>g <strong>the</strong> concentration of salt <strong>in</strong> <strong>the</strong> water on that<br />
side. If a reversal of this flow is desired, <strong>the</strong> pressure on <strong>the</strong> salt water side is <strong>in</strong>creased and now <strong>the</strong><br />
flow occurs from <strong>the</strong> salt side to <strong>the</strong> pure side. This reversal mechanism gives <strong>the</strong> process its name and<br />
accomplishes <strong>the</strong> desalt<strong>in</strong>g of <strong>the</strong> feedwater without a change of phase (i.e. <strong>the</strong> water is not required to<br />
be converted <strong>in</strong>to steam prior to its desal<strong>in</strong>ation).<br />
As can now be perceived, <strong>the</strong> design of <strong>the</strong> membrane, its chemical composition and its physical<br />
characteristics (e.g. pore size, etc.) must be carefully balanced <strong>for</strong> <strong>the</strong> <strong>in</strong>tended job. Most desal<strong>in</strong>ationplant<br />
RO membranes are designed <strong>for</strong> <strong>the</strong> flow of water across <strong>the</strong>m. It must be realized that s<strong>in</strong>ce this<br />
is a specific design balance predicated on <strong>the</strong> passage of water, dissolved compounds which are<br />
chemically similar to water will also pass readily through <strong>the</strong> membrane, s<strong>in</strong>ce <strong>the</strong>y will <strong>in</strong>teract with <strong>the</strong><br />
membrane <strong>in</strong> a similar manner. If <strong>the</strong> composition of <strong>the</strong> feedwater is such that <strong>the</strong>se compounds are<br />
present <strong>in</strong> excessive quantities, <strong>the</strong>n pre-treattnent of <strong>the</strong> feedwater becomes a must. It is <strong>for</strong> this reason<br />
that detailed analyses of <strong>the</strong> feedwater are required, <strong>in</strong>clud<strong>in</strong>g tests <strong>for</strong> biological compounds that would<br />
affect <strong>the</strong> TOC, BOD, COD, etc., as well as tests <strong>for</strong> colloidal matter that may entra<strong>in</strong> such compounds.<br />
As to <strong>the</strong> rest of <strong>the</strong> features of an RO Process unit, <strong>the</strong>y are quite similar to <strong>the</strong> EDR unit, <strong>in</strong> that<br />
feedwater needs to be pre-treated, br<strong>in</strong>e discharge is recycled <strong>for</strong> greater efficiency, temperature and<br />
pressure are carefully controlled (albeit pressures <strong>in</strong> an RO Process are altoge<strong>the</strong>r higher than those <strong>in</strong><br />
an EDR Process unit) and <strong>the</strong> ease of operation is sensitive to <strong>the</strong> sal<strong>in</strong>ity of <strong>the</strong> feedwater.<br />
Today's RO units can handle sal<strong>in</strong>ities up to those found <strong>in</strong> seawater (35,000 ppm IDS), although <strong>the</strong>ir<br />
best per<strong>for</strong>mance is usually realized when sal<strong>in</strong>ities are no greater than some 15,000 ppm IDS. Part of<br />
this constra<strong>in</strong>t arises from <strong>the</strong> fact that, after a period of cont<strong>in</strong>uous operation, <strong>the</strong> membranes suffer from<br />
"concentration polarization", a phenomenon <strong>in</strong> which <strong>the</strong> salt concentration on <strong>the</strong> face of <strong>the</strong> membrane<br />
exposed to <strong>the</strong> feedwater side is greater than that <strong>in</strong> <strong>the</strong> feedwater itself. This <strong>the</strong>n requires periodic<br />
flush<strong>in</strong>g and dilution procedures that can raise overall operat<strong>in</strong>g costs significantly, especially <strong>in</strong> those<br />
cases where membranes are required to ma<strong>in</strong>ta<strong>in</strong> high water flows per unit area. Recent advances <strong>in</strong>