Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
O O<br />
S<br />
HN<br />
S<br />
Application of Scor<strong>in</strong>g Functions <strong>in</strong> Virtual Screen<strong>in</strong>g 73<br />
O<br />
O<br />
S<br />
NH2 O<br />
S S S<br />
O<br />
O<br />
O<br />
S<br />
NH2 O<br />
O<br />
S<br />
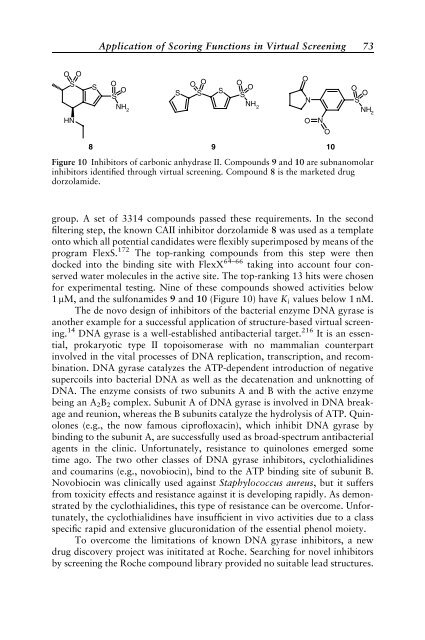
NH2 group. A set of 3314 compounds passed these requirements. In the second<br />
filter<strong>in</strong>g step, the known CAII <strong>in</strong>hibitor dorzolamide 8 was used as a template<br />
onto which all potential candidates were flexibly superimposed by means of the<br />
program FlexS. 172 The top-rank<strong>in</strong>g compounds from this step were then<br />
docked <strong>in</strong>to the b<strong>in</strong>d<strong>in</strong>g site with FlexX 64–66 tak<strong>in</strong>g <strong>in</strong>to account four conserved<br />
water molecules <strong>in</strong> the active site. The top-rank<strong>in</strong>g 13 hits were chosen<br />
for experimental test<strong>in</strong>g. N<strong>in</strong>e of these compounds showed activities below<br />
1 mM, and the sulfonamides 9 and 10 (Figure 10) have Ki values below 1 nM.<br />
The de novo design of <strong>in</strong>hibitors of the bacterial enzyme DNA gyrase is<br />
another example for a successful application of structure-based virtual screen<strong>in</strong>g.<br />
14 DNA gyrase is a well-established antibacterial target. 216 It is an essential,<br />
prokaryotic type II topoisomerase with no mammalian counterpart<br />
<strong>in</strong>volved <strong>in</strong> the vital processes of DNA replication, transcription, and recomb<strong>in</strong>ation.<br />
DNA gyrase catalyzes the ATP-dependent <strong>in</strong>troduction of negative<br />
supercoils <strong>in</strong>to bacterial DNA as well as the decatenation and unknott<strong>in</strong>g of<br />
DNA. The enzyme consists of two subunits A and B with the active enzyme<br />
be<strong>in</strong>g an A2B2 complex. Subunit A of DNA gyrase is <strong>in</strong>volved <strong>in</strong> DNA breakage<br />
and reunion, whereas the B subunits catalyze the hydrolysis of ATP. Qu<strong>in</strong>olones<br />
(e.g., the now famous ciprofloxac<strong>in</strong>), which <strong>in</strong>hibit DNA gyrase by<br />
b<strong>in</strong>d<strong>in</strong>g to the subunit A, are successfully used as broad-spectrum antibacterial<br />
agents <strong>in</strong> the cl<strong>in</strong>ic. Unfortunately, resistance to qu<strong>in</strong>olones emerged some<br />
time ago. The two other classes of DNA gyrase <strong>in</strong>hibitors, cyclothialid<strong>in</strong>es<br />
and coumar<strong>in</strong>s (e.g., novobioc<strong>in</strong>), b<strong>in</strong>d to the ATP b<strong>in</strong>d<strong>in</strong>g site of subunit B.<br />
Novobioc<strong>in</strong> was cl<strong>in</strong>ically used aga<strong>in</strong>st Staphylococcus aureus, but it suffers<br />
from toxicity effects and resistance aga<strong>in</strong>st it is develop<strong>in</strong>g rapidly. As demonstrated<br />
by the cyclothialid<strong>in</strong>es, this type of resistance can be overcome. Unfortunately,<br />
the cyclothialid<strong>in</strong>es have <strong>in</strong>sufficient <strong>in</strong> vivo activities due to a class<br />
specific rapid and extensive glucuronidation of the essential phenol moiety.<br />
To overcome the limitations of known DNA gyrase <strong>in</strong>hibitors, a new<br />
drug discovery project was <strong>in</strong>ititated at Roche. Search<strong>in</strong>g for novel <strong>in</strong>hibitors<br />
by screen<strong>in</strong>g the Roche compound library provided no suitable lead structures.<br />
O<br />
N<br />
O<br />
N<br />
O<br />
8 9 10<br />
Figure 10 Inhibitors of carbonic anhydrase II. Compounds 9 and 10 are subnanomolar<br />
<strong>in</strong>hibitors identified through virtual screen<strong>in</strong>g. Compound 8 is the marketed drug<br />
dorzolamide.