Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Vn (1 + cos(nω + γ))<br />
2<br />
K θ (θ − θ 0 ) 2<br />
from gas-phase ab <strong>in</strong>itio calculations. Enhanced charge values are a means<br />
of account<strong>in</strong>g for the strong polarization of electron distributions by the electric<br />
fields of the other particles <strong>in</strong> a condensed phase environment. The<br />
enhanced charges are obta<strong>in</strong>ed either through explicit parameterization 4,5 or<br />
by us<strong>in</strong>g charges obta<strong>in</strong>ed via quantum chemical methods that are known to<br />
overestimate charge values. 6 Although the enhanced charge values treat polarization<br />
<strong>in</strong> an effective way, they cannot correctly reflect the dependence of<br />
charge distributions on the system’s state, nor can they respond dynamically<br />
to fluctuations <strong>in</strong> the electric field due to molecular motion. The average electric<br />
field, and therefore the charge distribution and dipole moment, will<br />
depend on the physical state and composition of the system. For example, a<br />
molecule <strong>in</strong> a solution with a high ionic strength may feel a field different<br />
from a molecule <strong>in</strong> a pure solvent; even <strong>in</strong> the bulk liquid state, the polarization<br />
of a water molecule will depend on the density, and thus on the system’s<br />
temperature and pressure. In addition, conformational changes may <strong>in</strong>fluence<br />
the charge distribution of a molecule. 7–13 Molecular motions <strong>in</strong> the system will<br />
result <strong>in</strong> conformational changes and fluctuations <strong>in</strong> the electric field, caus<strong>in</strong>g<br />
the electrostatic distribution to change on a subpicosecond time scale. Treat<strong>in</strong>g<br />
these effects requires a polarizable model.<br />
POLARIZABLE POINT DIPOLES<br />
C<br />
O<br />
H H<br />
O<br />
H<br />
N<br />
K B (r − r 0 ) 2<br />
(q iq j /r ij ) + 4 ij<br />
Polarizable Po<strong>in</strong>t Dipoles 91<br />
One method for treat<strong>in</strong>g polarizability is to add po<strong>in</strong>t <strong>in</strong>ducible dipoles<br />
on some or all atomic sites. This polarizable po<strong>in</strong>t dipoles (PPD) method has<br />
been applied to a wide variety of atomic and molecular systems, rang<strong>in</strong>g from<br />
noble gases to water to prote<strong>in</strong>s. The dipole moment, l i, <strong>in</strong>duced on a site i is<br />
∋<br />
σ ij<br />
r ij<br />
12 σ 6<br />
ij<br />
−<br />
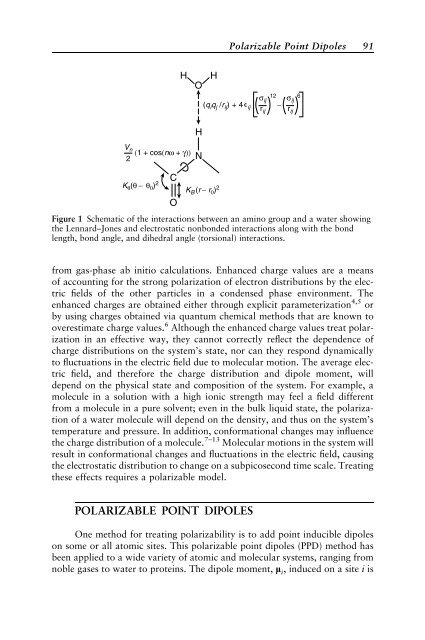
rij Figure 1 Schematic of the <strong>in</strong>teractions between an am<strong>in</strong>o group and a water show<strong>in</strong>g<br />
the Lennard–Jones and electrostatic nonbonded <strong>in</strong>teractions along with the bond<br />
length, bond angle, and dihedral angle (torsional) <strong>in</strong>teractions.