- Page 1 and 2:
Reviews in Computational Chemistry
- Page 3 and 4:
Kenny B. Lipkowitz Department of Ch
- Page 5 and 6:
vi Preface three-dimensional struct
- Page 7 and 8:
viii Preface some descriptors and i
- Page 9 and 10:
Epilogue and Dedication My associat
- Page 11 and 12:
Contents 1. Clustering Methods and
- Page 13 and 14:
Contents xv Electron Transfer in Po
- Page 15 and 16:
Contributors John M. Barnard, Barna
- Page 17 and 18:
Contributors to Previous Volumes *
- Page 19 and 20:
Volume 3 (1992) Tamar Schlick, Opti
- Page 21 and 22:
Volume 7 (1996) Geoffrey M. Downs a
- Page 23 and 24:
Volume 11 (1997) Mark A. Murcko, Re
- Page 25 and 26:
T. Daniel Crawford* and Henry F. Sc
- Page 27 and 28:
Topics Covered in Volumes 1-18 * Ab
- Page 29 and 30:
Reviews in Computational Chemistry
- Page 31 and 32:
2 Clustering Methods and Their Uses
- Page 33 and 34:
4 Clustering Methods and Their Uses
- Page 35 and 36:
6 Clustering Methods and Their Uses
- Page 37 and 38:
8 Clustering Methods and Their Uses
- Page 39 and 40:
10 Clustering Methods and Their Use
- Page 41 and 42:
12 Clustering Methods and Their Use
- Page 43 and 44:
14 Clustering Methods and Their Use
- Page 45 and 46: 16 Clustering Methods and Their Use
- Page 47 and 48: 18 Clustering Methods and Their Use
- Page 49 and 50: 20 Clustering Methods and Their Use
- Page 51 and 52: 22 Clustering Methods and Their Use
- Page 53 and 54: 24 Clustering Methods and Their Use
- Page 55 and 56: 26 Clustering Methods and Their Use
- Page 57 and 58: 28 Clustering Methods and Their Use
- Page 59 and 60: 30 Clustering Methods and Their Use
- Page 61 and 62: 32 Clustering Methods and Their Use
- Page 63 and 64: 34 Clustering Methods and Their Use
- Page 65 and 66: 36 Clustering Methods and Their Use
- Page 67 and 68: 38 Clustering Methods and Their Use
- Page 69 and 70: 40 Clustering Methods and Their Use
- Page 71 and 72: 42 The Use of Scoring Functions in
- Page 73 and 74: 44 The Use of Scoring Functions in
- Page 75 and 76: 46 The Use of Scoring Functions in
- Page 77 and 78: 48 The Use of Scoring Functions in
- Page 79 and 80: Table 1 Reference List for the Most
- Page 81 and 82: 52 The Use of Scoring Functions in
- Page 83 and 84: 54 The Use of Scoring Functions in
- Page 85 and 86: 56 The Use of Scoring Functions in
- Page 87 and 88: 58 The Use of Scoring Functions in
- Page 89 and 90: 60 The Use of Scoring Functions in
- Page 91 and 92: 62 The Use of Scoring Functions in
- Page 93 and 94: 64 The Use of Scoring Functions in
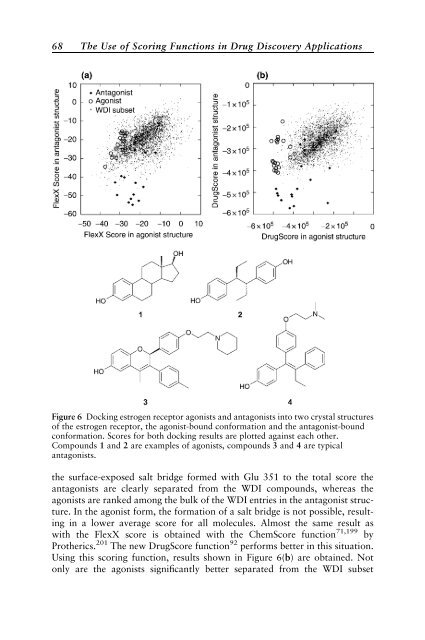
- Page 95: 66 The Use of Scoring Functions in
- Page 99 and 100: 70 The Use of Scoring Functions in
- Page 101 and 102: 72 The Use of Scoring Functions in
- Page 103 and 104: 74 The Use of Scoring Functions in
- Page 105 and 106: 76 The Use of Scoring Functions in
- Page 107 and 108: 78 The Use of Scoring Functions in
- Page 109 and 110: 80 The Use of Scoring Functions in
- Page 111 and 112: 82 The Use of Scoring Functions in
- Page 113 and 114: 84 The Use of Scoring Functions in
- Page 115 and 116: 86 The Use of Scoring Functions in
- Page 117 and 118: CHAPTER 3 Potentials and Algorithms
- Page 119 and 120: Vn (1 + cos(nω + γ)) 2 K θ (θ
- Page 121 and 122: are modified by their environment w
- Page 123 and 124: Table 1 Polarizability Parameters f
- Page 125 and 126: The polarizable point dipole models
- Page 127 and 128: two to three orders of magnitude sl
- Page 129 and 130: M i z i + q i d i k i and shell cha
- Page 131 and 132: Shell Models 103 on estimates of sh
- Page 133 and 134: minimization can be replaced by mor
- Page 135 and 136: The energy required to create a cha
- Page 137 and 138: for all i ði:e:; 8 iÞ: @U @qi l
- Page 139 and 140: where we have used q Cl ¼ qNa. The
- Page 141 and 142: Electronegativity Equalization Mode
- Page 143 and 144: Electronegativity Equalization Mode
- Page 145 and 146: of N molecules is taken as a Hartre
- Page 147 and 148:
is treated using variable charges.
- Page 149 and 150:
water have been developed, includin
- Page 151 and 152:
Applications 123 classical and rigi
- Page 153 and 154:
developing polarizable models. A va
- Page 155 and 156:
Comparison of the Polarization Mode
- Page 157 and 158:
negligible errors in such propertie
- Page 159 and 160:
ecome significant at field strength
- Page 161 and 162:
noteworthy in this regard because t
- Page 163 and 164:
References 135 9. P. Cieplak and P.
- Page 165 and 166:
References 137 48. E. L. Pollock an
- Page 167 and 168:
References 139 Computational Chemis
- Page 169 and 170:
References 141 139. J. Hinze and H.
- Page 171 and 172:
References 143 178. J. J. P. Stewar
- Page 173 and 174:
References 145 216. M. W. Mahoney a
- Page 175 and 176:
CHAPTER 4 New Developments in the T
- Page 177 and 178:
Introduction 149 applications). For
- Page 179 and 180:
FCWD(∆E ) FCWD(0) ∆E = 0 ∆E
- Page 181 and 182:
Introduction 153 Equations [6]-[12]
- Page 183 and 184:
Paradigm of Free Energy Surfaces 15
- Page 185 and 186:
Paradigm of Free Energy Surfaces 15
- Page 187 and 188:
In Eqs. [18] and [19], F0i is the e
- Page 189 and 190:
where ‘‘þ’’ and ‘‘ ’
- Page 191 and 192:
is, however, small for the usual co
- Page 193 and 194:
solute-solvent coupling through the
- Page 195 and 196:
where Z is the electrode overpotent
- Page 197 and 198:
energy surfaces of ET. 33,50-56 It
- Page 199 and 200:
This result indicates a fundamental
- Page 201 and 202:
βF i (X ) 40 20 0 −20 1 2 βλ 1
- Page 203 and 204:
Table 1 Main Features of the Two-Pa
- Page 205 and 206:
and Hss ¼ U rep ss 1 2 X j;k ðmj
- Page 207 and 208:
λ i /eV 4 3 2 1 0 0 10 20 30 40 Bo
- Page 209 and 210:
the width/Stokes shift relation (Eq
- Page 211 and 212:
Table 3 Mapping of the Q Model on S
- Page 213 and 214:
This situation is of course not sat
- Page 215 and 216:
F ± (Y ad )/λ I 0.8 0.4 0 −0.4
- Page 217 and 218:
it by choosing the GMH basis set 7
- Page 219 and 220:
Anharmonic higher order terms gain
- Page 221 and 222:
of the radiation is the perturbatio
- Page 223 and 224:
individual vibrational excitations
- Page 225 and 226:
m 12 /D 6 5.5 5 4.5 4 3.5 17 18 19
- Page 227 and 228:
In Eq. [144], the coordinates Y km
- Page 229 and 230:
ε/M −1 cm −1 4000 2000 0 analy
- Page 231 and 232:
βσ 2 /10 3 cm −1 14 12 10 8 Opt
- Page 233 and 234:
0.2 0.1 0 12 16 20 24 28 32 The app
- Page 235 and 236:
References 207 7. M. D. Newton, Adv
- Page 237 and 238:
References 209 59. R. Kubo and Y. T