Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
204 Charge-Transfer Reactions <strong>in</strong> Condensed Phases<br />
polarizability of the solute, a0i, treated as <strong>in</strong>put available from experiment or<br />
<strong>in</strong>dependent calculations, is thus split <strong>in</strong>to the polarizability from the 1 $ 2<br />
transition and the component a0i from all other transitions. The solvent effect<br />
on the transition between the states 1 and 2 then <strong>in</strong>cludes three components:<br />
(1) solvation of the fixed charges (dipole moments) of the chromophore, (2)<br />
self-polarization of the solute’s electronic cloud due to polarizability, and<br />
(3) change <strong>in</strong> the electronic occupation numbers <strong>in</strong>duced by the off-diagonal<br />
coupl<strong>in</strong>g of the transition dipole to the solvent field.<br />
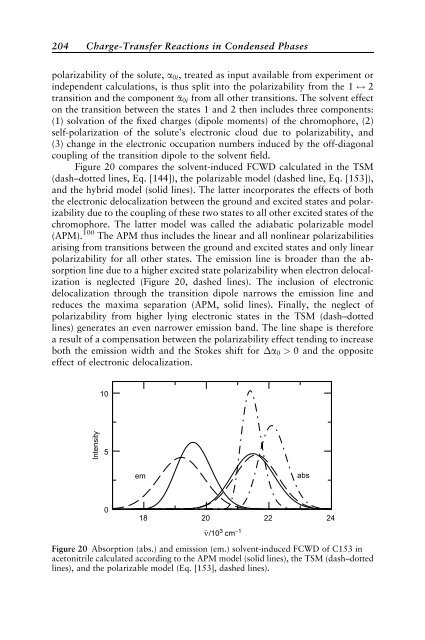
Figure 20 compares the solvent-<strong>in</strong>duced FCWD calculated <strong>in</strong> the TSM<br />
(dash–dotted l<strong>in</strong>es, Eq. [144]), the polarizable model (dashed l<strong>in</strong>e, Eq. [153]),<br />
and the hybrid model (solid l<strong>in</strong>es). The latter <strong>in</strong>corporates the effects of both<br />
the electronic delocalization between the ground and excited states and polarizability<br />
due to the coupl<strong>in</strong>g of these two states to all other excited states of the<br />
chromophore. The latter model was called the adiabatic polarizable model<br />
(APM). 100 The APM thus <strong>in</strong>cludes the l<strong>in</strong>ear and all nonl<strong>in</strong>ear polarizabilities<br />
aris<strong>in</strong>g from transitions between the ground and excited states and only l<strong>in</strong>ear<br />
polarizability for all other states. The emission l<strong>in</strong>e is broader than the absorption<br />
l<strong>in</strong>e due to a higher excited state polarizability when electron delocalization<br />
is neglected (Figure 20, dashed l<strong>in</strong>es). The <strong>in</strong>clusion of electronic<br />
delocalization through the transition dipole narrows the emission l<strong>in</strong>e and<br />
reduces the maxima separation (APM, solid l<strong>in</strong>es). F<strong>in</strong>ally, the neglect of<br />
polarizability from higher ly<strong>in</strong>g electronic states <strong>in</strong> the TSM (dash–dotted<br />
l<strong>in</strong>es) generates an even narrower emission band. The l<strong>in</strong>e shape is therefore<br />
a result of a compensation between the polarizability effect tend<strong>in</strong>g to <strong>in</strong>crease<br />
both the emission width and the Stokes shift for a0 > 0 and the opposite<br />
effect of electronic delocalization.<br />
Intensity<br />
10<br />
5<br />
0<br />
em abs<br />
<strong>18</strong> 20 22 24<br />
−<br />
ν /10 3 cm −1<br />
Figure 20 Absorption (abs.) and emission (em.) solvent-<strong>in</strong>duced FCWD of C153 <strong>in</strong><br />
acetonitrile calculated accord<strong>in</strong>g to the APM model (solid l<strong>in</strong>es), the TSM (dash–dotted<br />
l<strong>in</strong>es), and the polarizable model (Eq. [153], dashed l<strong>in</strong>es).