Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
148 Charge-Transfer Reactions <strong>in</strong> Condensed Phases<br />
reviews, but rather to highlight some very recent developments <strong>in</strong> the field that<br />
have not been reviewed. This chapter will provide the reader with a step-bystep<br />
statistical mechanical buildup of the theoretical mach<strong>in</strong>ery currently<br />
employed <strong>in</strong> ET research. By virtue of the ‘‘frontier’’ nature of this material,<br />
many traditional subjects of ET studies are not covered here. The reader will<br />
be referred to previous reviews whenever possible, but many excellent contributions<br />
are not directly cited.<br />
This chapter concerns the energetics of charge-transfer (CT) reactions.<br />
We will not discuss subjects deal<strong>in</strong>g with nuclear dynamical effects on CT<br />
k<strong>in</strong>etics. 2–4 The more specialized topic of employ<strong>in</strong>g the liquid-state theories<br />
to calculate the solvation component of the reorganization parameters 5 is<br />
not considered here. We concentrate <strong>in</strong>stead on the general procedure of the<br />
statistical mechanical analysis of the activation barrier to CT, as well as on its<br />
connection to optical spectroscopy. S<strong>in</strong>ce the very beg<strong>in</strong>n<strong>in</strong>g of ET research, 6<br />
steady-state optical spectroscopy has been the major source of reliable <strong>in</strong>formation<br />
about the activation barrier and preexponential factor for the ET rate.<br />
The ma<strong>in</strong> focus <strong>in</strong> this chapter is therefore on the connection between the statistical<br />
analysis of the reaction activation barrier to the steady-state optical<br />
band shape.<br />
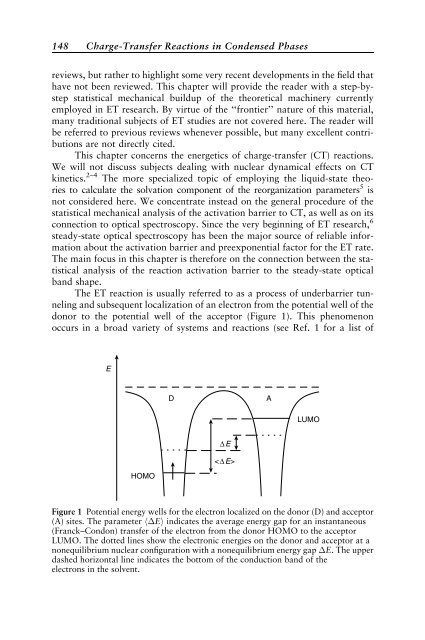
The ET reaction is usually referred to as a process of underbarrier tunnel<strong>in</strong>g<br />
and subsequent localization of an electron from the potential well of the<br />
donor to the potential well of the acceptor (Figure 1). This phenomenon<br />
occurs <strong>in</strong> a broad variety of systems and reactions (see Ref. 1 for a list of<br />
E<br />
HOMO<br />
D A<br />
∆E<br />
<br />
LUMO<br />
Figure 1 Potential energy wells for the electron localized on the donor (D) and acceptor<br />
(A) sites. The parameter h Ei <strong>in</strong>dicates the average energy gap for an <strong>in</strong>stantaneous<br />
(Franck–Condon) transfer of the electron from the donor HOMO to the acceptor<br />
LUMO. The dotted l<strong>in</strong>es show the electronic energies on the donor and acceptor at a<br />
nonequilibrium nuclear configuration with a nonequilibrium energy gap E. The upper<br />
dashed horizontal l<strong>in</strong>e <strong>in</strong>dicates the bottom of the conduction band of the<br />
electrons <strong>in</strong> the solvent.