Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
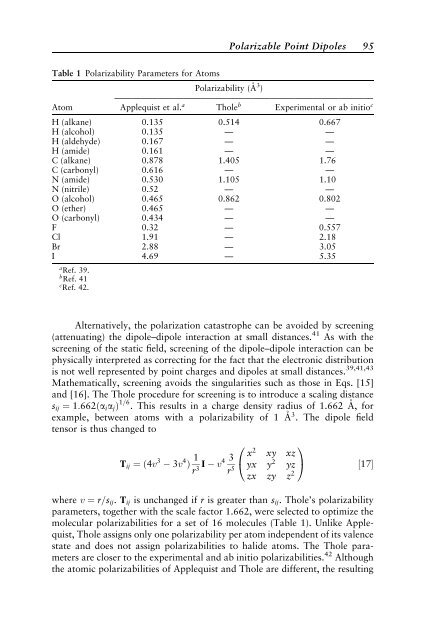
Table 1 Polarizability Parameters for Atoms<br />
Polarizability (A˚ 3<br />
)<br />
——————————————————————————————<br />
Atom Applequist et al. a Tholeb Experimental or ab <strong>in</strong>itioc H (alkane) 0.135 0.514 0.667<br />
H (alcohol) 0.135 — —<br />
H (aldehyde) 0.167 — —<br />
H (amide) 0.161 — —<br />
C (alkane) 0.878 1.405 1.76<br />
C (carbonyl) 0.616 — —<br />
N (amide) 0.530 1.105 1.10<br />
N (nitrile) 0.52 — —<br />
O (alcohol) 0.465 0.862 0.802<br />
O (ether) 0.465 — —<br />
O (carbonyl) 0.434 — —<br />
F 0.32 — 0.557<br />
Cl 1.91 — 2.<strong>18</strong><br />
Br 2.88 — 3.05<br />
I 4.69 — 5.35<br />
a Ref. 39.<br />
b Ref. 41<br />
c Ref. 42.<br />
Alternatively, the polarization catastrophe can be avoided by screen<strong>in</strong>g<br />
(attenuat<strong>in</strong>g) the dipole–dipole <strong>in</strong>teraction at small distances. 41 As with the<br />
screen<strong>in</strong>g of the static field, screen<strong>in</strong>g of the dipole–dipole <strong>in</strong>teraction can be<br />
physically <strong>in</strong>terpreted as correct<strong>in</strong>g for the fact that the electronic distribution<br />
is not well represented by po<strong>in</strong>t charges and dipoles at small distances. 39,41,43<br />
Mathematically, screen<strong>in</strong>g avoids the s<strong>in</strong>gularities such as those <strong>in</strong> Eqs. [15]<br />
and [16]. The Thole procedure for screen<strong>in</strong>g is to <strong>in</strong>troduce a scal<strong>in</strong>g distance<br />
sij ¼ 1:662ðaiajÞ 1=6 . This results <strong>in</strong> a charge density radius of 1.662 A˚ , for<br />
example, between atoms with a polarizability of 1 A˚ 3 . The dipole field<br />
tensor is thus changed to<br />
0<br />
1<br />
Tij ¼ð4v 3<br />
3v 4 Þ 1 3<br />
I v4<br />
r3 r5 Polarizable Po<strong>in</strong>t Dipoles 95<br />
x2 xy xz<br />
@<br />
A ½17Š<br />
yx y 2 yz<br />
zx zy z 2<br />
where v ¼ r=sij. Tij is unchanged if r is greater than sij. Thole’s polarizability<br />
parameters, together with the scale factor 1.662, were selected to optimize the<br />
molecular polarizabilities for a set of 16 molecules (Table 1). Unlike Applequist,<br />
Thole assigns only one polarizability per atom <strong>in</strong>dependent of its valence<br />
state and does not assign polarizabilities to halide atoms. The Thole parameters<br />
are closer to the experimental and ab <strong>in</strong>itio polarizabilities. 42 Although<br />
the atomic polarizabilities of Applequist and Thole are different, the result<strong>in</strong>g