Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
46 The Use of Scor<strong>in</strong>g Functions <strong>in</strong> Drug Discovery Applications<br />
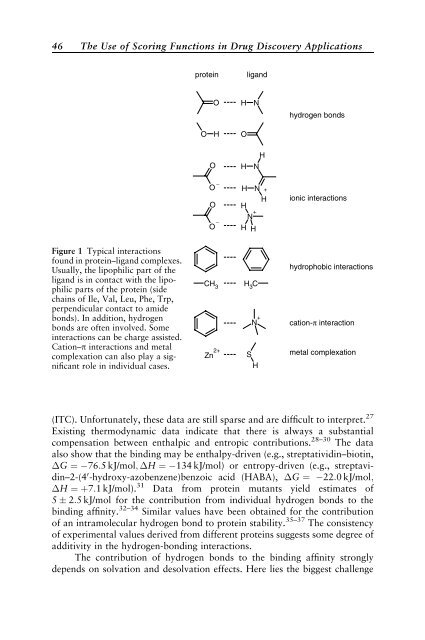
Figure 1 Typical <strong>in</strong>teractions<br />
found <strong>in</strong> prote<strong>in</strong>–ligand complexes.<br />
Usually, the lipophilic part of the<br />
ligand is <strong>in</strong> contact with the lipophilic<br />
parts of the prote<strong>in</strong> (side<br />
cha<strong>in</strong>s of Ile, Val, Leu, Phe, Trp,<br />
perpendicular contact to amide<br />
bonds). In addition, hydrogen<br />
bonds are often <strong>in</strong>volved. Some<br />
<strong>in</strong>teractions can be charge assisted.<br />
Cation–p <strong>in</strong>teractions and metal<br />
complexation can also play a significant<br />
role <strong>in</strong> <strong>in</strong>dividual cases.<br />
prote<strong>in</strong> ligand<br />
O H N<br />
O H O<br />
H<br />
O H N<br />
−<br />
O<br />
O<br />
−<br />
O<br />
CH 3<br />
Zn 2+<br />
H N +<br />
H<br />
+<br />
N<br />
H H<br />
H<br />
(ITC). Unfortunately, these data are still sparse and are difficult to <strong>in</strong>terpret. 27<br />
Exist<strong>in</strong>g thermodynamic data <strong>in</strong>dicate that there is always a substantial<br />
compensation between enthalpic and entropic contributions. 28–30 The data<br />
also show that the b<strong>in</strong>d<strong>in</strong>g may be enthalpy-driven (e.g., streptativid<strong>in</strong>–biot<strong>in</strong>,<br />
G ¼ 76:5 kJ/mol; H ¼ 134 kJ/mol) or entropy-driven (e.g., streptavid<strong>in</strong>–2-(4<br />
0 -hydroxy-azobenzene)benzoic acid (HABA), G ¼ 22:0 kJ/mol;<br />
H ¼þ7:1 kJ/mol). 31 Data from prote<strong>in</strong> mutants yield estimates of<br />
5 2:5 kJ/mol for the contribution from <strong>in</strong>dividual hydrogen bonds to the<br />
b<strong>in</strong>d<strong>in</strong>g aff<strong>in</strong>ity. 32–34 Similar values have been obta<strong>in</strong>ed for the contribution<br />
of an <strong>in</strong>tramolecular hydrogen bond to prote<strong>in</strong> stability. 35–37 The consistency<br />
of experimental values derived from different prote<strong>in</strong>s suggests some degree of<br />
additivity <strong>in</strong> the hydrogen-bond<strong>in</strong>g <strong>in</strong>teractions.<br />
The contribution of hydrogen bonds to the b<strong>in</strong>d<strong>in</strong>g aff<strong>in</strong>ity strongly<br />
depends on solvation and desolvation effects. Here lies the biggest challenge<br />
C<br />
H 3<br />
N +<br />
S<br />
H<br />
hydrogen bonds<br />
ionic <strong>in</strong>teractions<br />
hydrophobic <strong>in</strong>teractions<br />
cation-π <strong>in</strong>teraction<br />
metal complexation