Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
Reviews in Computational Chemistry Volume 18
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
prote<strong>in</strong><br />
N<br />
H<br />
H O H<br />
prote<strong>in</strong><br />
CH 3<br />
H O H<br />
H<br />
O<br />
N<br />
H<br />
H<br />
+ +<br />
O<br />
O<br />
ligand prote<strong>in</strong>-ligand<br />
complex<br />
H<br />
O<br />
H<br />
CH3 + +<br />
CH 3<br />
H<br />
O<br />
H<br />
H O H<br />
H<br />
O<br />
H<br />
H O H<br />
<strong>in</strong> the quantitative treatment of prote<strong>in</strong>–ligand <strong>in</strong>teractions: provid<strong>in</strong>g an<br />
accurate description of the role of water molecules (Figure 2). It has been<br />
shown, by compar<strong>in</strong>g the b<strong>in</strong>d<strong>in</strong>g aff<strong>in</strong>ities of ligand pairs differ<strong>in</strong>g by just<br />
one hydrogen bond, that the existence of an <strong>in</strong>dividual hydrogen bond can<br />
even be adverse to b<strong>in</strong>d<strong>in</strong>g. 38 Charge-assisted hydrogen bonds are stronger<br />
than neutral ones, but this enhancement <strong>in</strong> b<strong>in</strong>d<strong>in</strong>g is paid for by higher<br />
desolvation penalties. The electrostatic <strong>in</strong>teraction of an exposed salt bridge<br />
is worth as much as a neutral hydrogen bond (5 1 kJ/mol accord<strong>in</strong>g to<br />
Ref. 39), and the same <strong>in</strong>teraction <strong>in</strong> the <strong>in</strong>terior of a prote<strong>in</strong> can be significantly<br />
larger. 40<br />
The experimental determ<strong>in</strong>ation of H and S sometimes yields surpris<strong>in</strong>g<br />
results, as, for example, <strong>in</strong> the thermodynamics of hydrogen-bond formation<br />
<strong>in</strong> the complex of FK506 or rapamyc<strong>in</strong> with FK506-b<strong>in</strong>d<strong>in</strong>g prote<strong>in</strong><br />
(FKBP). 34 B<strong>in</strong>d<strong>in</strong>g to the wild-type and to the mutant Tyr 82 ! Phe 82 was<br />
CH 3<br />
ligand prote<strong>in</strong>-ligand<br />
complex<br />
Introduction 47<br />
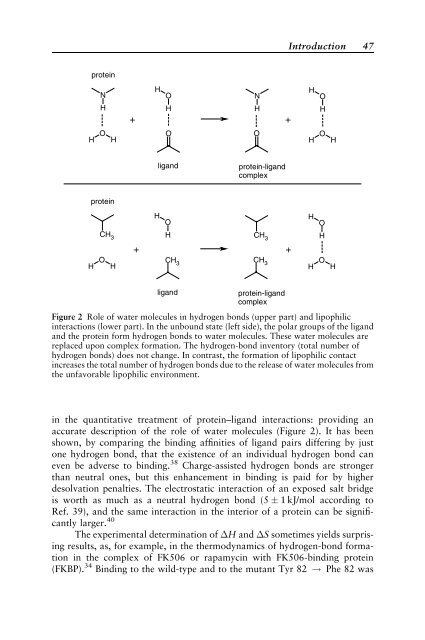
Figure 2 Role of water molecules <strong>in</strong> hydrogen bonds (upper part) and lipophilic<br />
<strong>in</strong>teractions (lower part). In the unbound state (left side), the polar groups of the ligand<br />
and the prote<strong>in</strong> form hydrogen bonds to water molecules. These water molecules are<br />
replaced upon complex formation. The hydrogen-bond <strong>in</strong>ventory (total number of<br />
hydrogen bonds) does not change. In contrast, the formation of lipophilic contact<br />
<strong>in</strong>creases the total number of hydrogen bonds due to the release of water molecules from<br />
the unfavorable lipophilic environment.