Introduction to Soil Chemistry
Introduction to Soil Chemistry
Introduction to Soil Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
extraction 131<br />
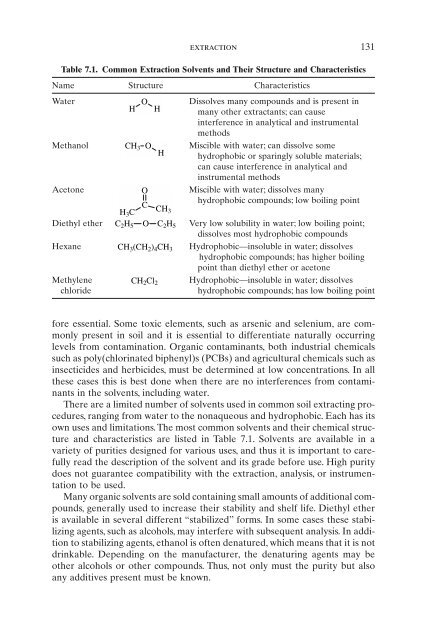
Table 7.1. Common Extraction Solvents and Their Structure and Characteristics<br />
Name Structure Characteristics<br />
Water<br />
H<br />
O<br />
H<br />
Dissolves many compounds and is present in<br />
many other extractants; can cause<br />
interference in analytical and instrumental<br />
methods<br />
Methanol CH3 O<br />
H<br />
Miscible with water; can dissolve some<br />
hydrophobic or sparingly soluble materials;<br />
can cause interference in analytical and<br />
instrumental methods<br />
Ace<strong>to</strong>ne O Miscible with water; dissolves many<br />
H3C C<br />
CH3 hydrophobic compounds; low boiling point<br />
Diethyl ether C2H5 O C2H5 Very low solubility in water; low boiling point;<br />
dissolves most hydrophobic compounds<br />
Hexane CH3(CH2) 4CH3 Hydrophobic—insoluble in water; dissolves<br />
hydrophobic compounds; has higher boiling<br />
point than diethyl ether or ace<strong>to</strong>ne<br />
Methylene<br />
chloride<br />
CH2Cl2 Hydrophobic—insoluble in water; dissolves<br />
hydrophobic compounds; has low boiling point<br />
fore essential. Some <strong>to</strong>xic elements, such as arsenic and selenium, are commonly<br />
present in soil and it is essential <strong>to</strong> differentiate naturally occurring<br />
levels from contamination. Organic contaminants, both industrial chemicals<br />
such as poly(chlorinated biphenyl)s (PCBs) and agricultural chemicals such as<br />
insecticides and herbicides, must be determined at low concentrations. In all<br />
these cases this is best done when there are no interferences from contaminants<br />
in the solvents, including water.<br />
There are a limited number of solvents used in common soil extracting procedures,<br />
ranging from water <strong>to</strong> the nonaqueous and hydrophobic. Each has its<br />
own uses and limitations. The most common solvents and their chemical structure<br />
and characteristics are listed in Table 7.1. Solvents are available in a<br />
variety of purities designed for various uses, and thus it is important <strong>to</strong> carefully<br />
read the description of the solvent and its grade before use. High purity<br />
does not guarantee compatibility with the extraction, analysis, or instrumentation<br />
<strong>to</strong> be used.<br />
Many organic solvents are sold containing small amounts of additional compounds,<br />
generally used <strong>to</strong> increase their stability and shelf life. Diethyl ether<br />
is available in several different “stabilized” forms. In some cases these stabilizing<br />
agents, such as alcohols, may interfere with subsequent analysis. In addition<br />
<strong>to</strong> stabilizing agents, ethanol is often denatured, which means that it is not<br />
drinkable. Depending on the manufacturer, the denaturing agents may be<br />
other alcohols or other compounds. Thus, not only must the purity but also<br />
any additives present must be known.