- Page 1: Introduction to Soil Chemistry Anal
- Page 4 and 5: CHEMICAL ANALYSIS A SERIES OF MONOG
- Page 6 and 7: Copyright © 2005 by John Wiley & S
- Page 9 and 10: CONTENTS PREFACE xiii CHAPTER 1 SOI
- Page 11 and 12: contents ix 4.14. Conclusion 89 Pro
- Page 13: contents xi CHAPTER 10 SPECIATION 1
- Page 16 and 17: xiv preface Moisture levels can cha
- Page 19 and 20: CHAPTER 1 SOIL BASICS I MACROSCALE
- Page 21 and 22: soil basics i 3 Mollisol Ap 0 - 17.
- Page 23 and 24: soil basic i 5 Spodosol Oi 0 - 2.5
- Page 25 and 26: horizonation 7 or basic.Thus, the t
- Page 27 and 28: horizonation 9 Table 1.2. Major-Min
- Page 29 and 30: peds 11 As would be expected, only
- Page 31 and 32: peds 13 Figure 1.7. Peds isolated f
- Page 33 and 34: P soil color 15 B P = pores B = bri
- Page 35 and 36: soil naming 17 Under most condition
- Page 37 and 38: soil chemistry, analysis, and instr
- Page 39 and 40: ibliography 21 thus increased oxida
- Page 41 and 42: CHAPTER 2 SOIL BASICS II MICROSCOPI
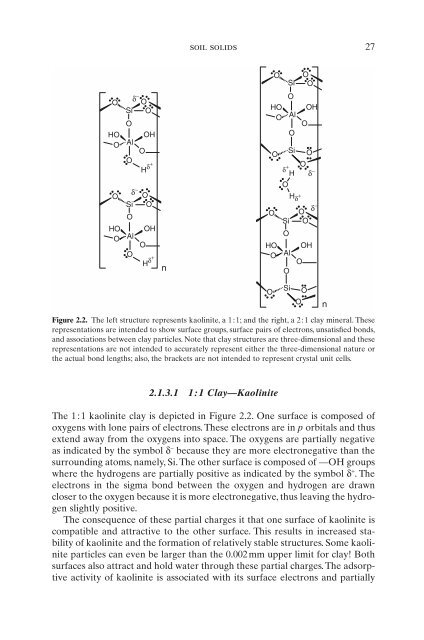
- Page 43: O O Si O O d - d H H + d + O soil s
- Page 47 and 48: O HO O O O HO O O Si O Al O Si O O
- Page 49 and 50: soil solids 31 the name taken from
- Page 51 and 52: onding considerations 33 The two op
- Page 53 and 54: surface features 35 Because differe
- Page 55 and 56: energy considerations 37 side-by-si
- Page 57 and 58: In Figure 2.5 the path to the right
- Page 59 and 60: H O H H O C Na +- O O H H O H H O H
- Page 61 and 62: eferences 43 2.7. Explain how the r
- Page 63 and 64: CHAPTER 3 SOIL BASICS III THE BIOLO
- Page 65 and 66: animals 47 C Figure 3.2. An ant col
- Page 67 and 68: plants 49 Grasses and other similar
- Page 69 and 70: plants 51 Rhizosphere Figure 3.4. I
- Page 71 and 72: microorganisms 53 Table 3.1. Design
- Page 73 and 74: microorganisms 55 microaerophilic,
- Page 75 and 76: ioorganic 57 activity may be increa
- Page 77 and 78: organic compounds 59 and may contai
- Page 79 and 80: organic compounds 61 Table 3.3. Fun
- Page 81 and 82: R 2C R organic compounds 63 O C - O
- Page 83 and 84: analysis 65 3.7.1. Analysis for Ani
- Page 85 and 86: problems 67 oxide by components in
- Page 87 and 88: eferences 69 6. Bais HP, Park S-W,
- Page 89 and 90: CHAPTER 4 SOIL BASICS IV THE SOIL A
- Page 91 and 92: water 73 The soil atmosphere also c
- Page 93 and 94: D C water 75 B Figure 4.3. A wide-d
- Page 95 and 96:
compounds in solution 77 tion is li
- Page 97 and 98:
+ NH4 + NH4 H H O + NH4 H H H + NH
- Page 99 and 100:
organic ions in solution 81 consequ
- Page 101 and 102:
distribution between soil solids an
- Page 103 and 104:
oxidative and reductive reactions i
- Page 105 and 106:
measuring soil water 87 soil is rep
- Page 107 and 108:
problems 89 blocks, and thermocoupl
- Page 109:
eferences 91 8. Bohn HL, McNeal BL,
- Page 112 and 113:
94 electrical measurements Figure 5
- Page 114 and 115:
96 electrical measurements 1200 100
- Page 116 and 117:
98 electrical measurements Referenc
- Page 118 and 119:
100 electrical measurements Standar
- Page 120 and 121:
102 Table 5.1. Ion-Selective Electr
- Page 122 and 123:
104 electrical measurements In addi
- Page 124 and 125:
106 electrical measurements along w
- Page 126 and 127:
108 electrical measurements cools w
- Page 128 and 129:
110 electrical measurements Science
- Page 130 and 131:
112 titrimetric measurement pH 13 1
- Page 132 and 133:
114 titrimetric measurement Table 6
- Page 134 and 135:
116 titrimetric measurement H 3 O +
- Page 136 and 137:
118 titrimetric measurement Because
- Page 138 and 139:
120 titrimetric measurement Soil Sa
- Page 140 and 141:
122 titrimetric measurement 6.6. NI
- Page 142 and 143:
124 titrimetric measurement - + X +
- Page 144 and 145:
126 titrimetric measurement REFEREN
- Page 146 and 147:
128 extraction Organic compounds, i
- Page 148 and 149:
130 extraction 3. What is the distr
- Page 150 and 151:
132 extraction All nonaqueous solve
- Page 152 and 153:
134 extraction Table 7.2. Character
- Page 154 and 155:
136 extraction Figure 7.3. Solution
- Page 156 and 157:
138 extraction Figure 7.5. A microw
- Page 158 and 159:
140 extraction serious interference
- Page 160 and 161:
142 extraction taining one to sever
- Page 162 and 163:
144 extraction Cohen DR, Shen XC, D
- Page 164 and 165:
146 extraction ultrasound; Applicat
- Page 166 and 167:
148 spectroscopy although technical
- Page 168 and 169:
150 spectroscopy Incident X-rays q
- Page 170 and 171:
152 spectroscopy with cellulose or
- Page 172 and 173:
154 spectroscopy extract components
- Page 174 and 175:
156 spectroscopy 8.6. ULTRAVIOLET A
- Page 176 and 177:
158 spectroscopy placed in the samp
- Page 178 and 179:
160 spectroscopy to scratch them. W
- Page 180 and 181:
162 spectroscopy Data Concentration
- Page 182 and 183:
164 spectroscopy these make analysi
- Page 184 and 185:
166 spectroscopy Table 8.1. Importa
- Page 186 and 187:
168 spectroscopy nance (NMR) spectr
- Page 188 and 189:
170 spectroscopy nitrogen containin
- Page 190 and 191:
172 spectroscopy 7. Bernick MB, Get
- Page 193 and 194:
CHAPTER 9 CHROMATOGRAPHY Chromatogr
- Page 195 and 196:
Mobile phase in abaybayby abaybayby
- Page 197 and 198:
gas chromatography 179 Figure 9.3.
- Page 199 and 200:
gas chromatography 181 Table 9.3. C
- Page 201 and 202:
gas chromatography 183 Gas tight sy
- Page 203 and 204:
high-performance liquid chromatogra
- Page 205 and 206:
thin-layer chromatography 187 They
- Page 207 and 208:
alization of components on a thin-l
- Page 209 and 210:
conclusion 191 then a pure sample o
- Page 211:
eferences 193 U.S. Environmental Pr
- Page 214 and 215:
196 speciation - Al H 2 O K H2O H2O
- Page 216 and 217:
198 speciation to consider all poss
- Page 218 and 219:
200 speciation many more species th
- Page 220 and 221:
202 speciation atomic absorption is
- Page 222 and 223:
204 speciation Table 10.2. Common O
- Page 224 and 225:
206 speciation reflecting the titra
- Page 226 and 227:
208 speciation Some oxyanions are s
- Page 228 and 229:
210 speciation 13. Wang J, Ashley K
- Page 230 and 231:
212 index Bioreactor, 124 Bioremedi
- Page 232 and 233:
214 index Gas chromatograph-mass sp
- Page 234 and 235:
216 index Path length, 159 Ped(s),
- Page 236 and 237:
218 index TMS, see Tetramethylsilan
- Page 238 and 239:
Vol. 34. Neutron Activation Analysi
- Page 240 and 241:
Vol. 117 Applications of Fluorescen