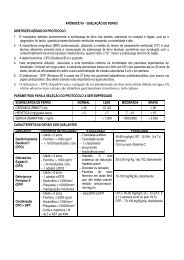
- Page 2 and 3: PROTOCOLS INSTITUTE OF HAEMATOLOGY
- Page 4 and 5: COORDINATION AND REVISION Clarisse
- Page 6 and 7: SUMMARY: PART I - CLINICAL PROTOCOL
- Page 8 and 9: 1.INICIAL CLINICAL EVALUATION SICKL
- Page 10 and 11: TREATMENTO AND IN ADULTS AT ANY AGE
- Page 12 and 13: SPLENECTOMY - INDICATIONS: (1) CHIL
- Page 14 and 15: OUTPATIENT TREATMENT: It is based o
- Page 16 and 17: ASPIRIN AND NSAID PARACETAMOL OPIOI
- Page 18 and 19: CONDUCTION: - Hospitalization - Com
- Page 20 and 21: ONE, TWO-DIMENSIONAL DOPPLER ECHOCA
- Page 22 and 23: Hemianopsia Homonymous deficit of v
- Page 24 and 25: TREATMENT OF CONVULSIVE EPISODES -
- Page 26 and 27: INITIAL ATTENDANCE TO PREGNANT WOME
- Page 28 and 29: - It may occurs acute thoracic synd
- Page 30 and 31: 3 Diet with potassium restriction 4
- Page 32 and 33: MICROALBUMINURIA Screening Patients
- Page 34 and 35: RECURRENCE OF PRIAPISM: - Pain Trea
- Page 36 and 37: SPECIAL CARES DEFERASIROX - The use
- Page 40 and 41: MAJOR AND INTERMIDIATE THALASSEMIA
- Page 42 and 43: HEREDIRATY SPHEROCYTOSIS CLINICAL E
- Page 44 and 45: G6PD DEFICIENCY INITIAL EVALUATION
- Page 46 and 47: LABS EXAMS TO DIAGNOSE APLASTIC ANE
- Page 48 and 49: SELFIMMUNE HEMOLYTIC ANEMIA CONCEPT
- Page 50 and 51: 2. Splenectomy: Vaccinate 14 days b
- Page 52 and 53: IMMUNE THROMBOCYTOPENIC PURPURA LAB
- Page 54 and 55: THROMBOTIC THROMBOCYTOPENIC PURPURA
- Page 56 and 57: HEMORRHAGIC SYNDROME INTRODUCTION H
- Page 58 and 59: MULTIDISCIPLINARY FOLLOW-UP OF HEMO
- Page 60 and 61: LYOPHILIZED CONCENTRATES: They are
- Page 62 and 63: TABLE 2: REPLACEMENT THERAPY TO PER
- Page 64 and 65: CONDUCTION IN CONFIRMED INTRACRANIA
- Page 66 and 67: Severe CET with neurological change
- Page 68 and 69: PHARMACOKINETICS OF CLOTTING FACTOR
- Page 70 and 71: INHIBITORS CLASSIFICATION The inhib
- Page 72 and 73: - Extended surgeries require increa
- Page 74 and 75: CLINICAL DIAGNOSIS: According to In
- Page 76 and 77: DDAVP TEST The “test dose” must
- Page 78 and 79: TREATMENT OF BLEEDING SITUATIONS SI
- Page 80 and 81: TABLE 6 - REPLACEMENT THERAPY IN LE
- Page 82 and 83: • Primary prophylaxis: controvers
- Page 84 and 85: CLINICAL AND LABORATORIAL FOLLOW-UP
- Page 86 and 87: MYELODISPLASIC SYNDROME LABORATORIA
- Page 88 and 89:
If EPO < 500 mU/ml : EPO (8000U 3x/
- Page 90 and 91:
ACUTE MYELOID LEUKEMIA LABORATORIAL
- Page 92 and 93:
LMA LMA M3 - non-M3 LMA Protocol LM
- Page 94 and 95:
Key: Volunteer to aggressive treatm
- Page 96 and 97:
Acute Promyelocytic Leukemia Transf
- Page 98 and 99:
BMP LMA IN CHILDREN AND TEENAGERS
- Page 100 and 101:
Ara-c dose reduction by age: Age in
- Page 102 and 103:
ATRA RECOMMENDATION: ATRA is starte
- Page 104 and 105:
- venous hydration - leukapheresis
- Page 106 and 107:
POLYCYTHEMIA VERA LABORATORIAL TEST
- Page 108 and 109:
TREATMENT - 2nd LINE - Indicated fo
- Page 110 and 111:
ACUTE LYMPHOID LEUKEMIA LABORATORIA
- Page 112 and 113:
RE-INDUCTION Phase I (weeks 1-4) -
- Page 114 and 115:
Cycles 2, 4, 6, 8 - Methotrexate 20
- Page 116 and 117:
PHASE 2 Infusion CPM in 1 hour 1000
- Page 118 and 119:
CURATIVE RADIOTHERAPY of CNS < 1 ye
- Page 120 and 121:
CHRONIC LYMPHOCYTIC LEUKEMIA LABORA
- Page 122 and 123:
fludarabine 25 mg/m VENOUS 2 D1-D3
- Page 124 and 125:
TRICHOLEUKEMIA LABORATORIAL TESTS -
- Page 126 and 127:
- age >45 years old - leukocytosis
- Page 128 and 129:
- Leukemia / T-Cell Lymphoma in Adu
- Page 130 and 131:
INDICATIONS FOR THE TREATMENT: Symp
- Page 132 and 133:
1 st line Chemotherapy R- CHOP (21
- Page 134 and 135:
CODOX- M/IVAC Cycles 1 and 3 Cyclop
- Page 136 and 137:
BLOCK A DEXA VO/IV 10 mg /m2 in 3 d
- Page 138 and 139:
- Surgical treatment is only indica
- Page 140 and 141:
LEUKEMIA / T-CELLS LYMPHOMA OF THE
- Page 142 and 143:
HN2 or PUVA (similar to the located
- Page 144 and 145:
MULTIPLE MYELOMA LABORATORIAL TESTS
- Page 146 and 147:
RESPONSE CRITERIA Full Response Abs
- Page 148 and 149:
WALDENSTRON MACROGLOBULINEMIA LABOR
- Page 150 and 151:
− If HLA-identical related donor
- Page 152 and 153:
TREATMENT PROTOCOL - ACUTE MYELOID
- Page 154 and 155:
TREATMENT PROTOCOL - MULTIPLE MYELO
- Page 156 and 157:
Post-autologous transplantation pro
- Page 158 and 159:
PART II - TRANSFUSIONAL PROTOCOLS A
- Page 160 and 161:
PRESERVATION AND CONSERVATION: The
- Page 162 and 163:
TRANSFUSION OF RED BLOOD CELLS, PLA
- Page 164 and 165:
FORMAL CONTRAINDICATIONS TO THE PLA
- Page 166 and 167:
1 - TRANSFUSION OF FROZEN FRESH PLA
- Page 168 and 169:
COMMUNICATION FORM OF CRYOPRECIPITA
- Page 170 and 171:
Volume to be = changed (*) VOLEMIA
- Page 172 and 173:
HEMOTHERAPIC PROTOCOL TO FALCIFORM
- Page 174 and 175:
5 - TRANSFUSIONAL CONDUCT AT THE SE
- Page 176 and 177:
CML - Subjects with symptoms relate
- Page 178 and 179:
- Aplastic anemia bearers - HIV pos
- Page 180 and 181:
Severe reaction: anaphylactic shock
- Page 182 and 183:
- Heart monitoring - Prescribe Furo
- Page 184 and 185:
CARES AT THE PLATELETS CONCENTRATE
- Page 186 and 187:
ATTACHMENT I - HEMATOLOGICAL CLINIC
- Page 188 and 189:
INFECTIOUS SPLENO MEGALIES IMMUNO L
- Page 190 and 191:
INVESTIGATION OF HEMORRHAGIC SYNDRO
- Page 192 and 193:
INVESTIGATION OF POLYGLOBULIAS: POL
- Page 194 and 195:
ATTACHMENT III - HEMATOLOGY SPECIAL
- Page 196 and 197:
IMMUNOPHENOTYPE PROFILE OF THE ACUT
- Page 198 and 199:
ATTACHMENT IV - PAIN APPROACH ROUTI
- Page 200 and 201:
If it is prescribed for more than 1
- Page 202 and 203:
HOW TO PRESCRIBE OPIOID: SPECIAL CA
- Page 204 and 205:
SPECIFICITIES OF EACH OPIATE (cont
- Page 206 and 207:
METHADONE Intravenous (*) Oral path
- Page 208 and 209:
Psychic Dependence or “addiction
- Page 210 and 211:
CROSS TOLERANCE - The subject must
- Page 212 and 213:
ATTACHMENT V - CHEMOTHERAPY SUBJECT
- Page 214 and 215:
ATTACHMENT VII - IRON CHELATION GEN
- Page 216 and 217:
10. G N N - DFP DFP-100 mg/kg/day d
- Page 218 and 219:
ATTACHMENT VIII - SEDATION IN CHILD
- Page 220 and 221:
224 NOTES _________________________
- Page 222 and 223:
224 NOTES _________________________
- Page 224:
Expresso Gráfica e Editora Ltda-me