Protocols - Hemorio
Protocols - Hemorio
Protocols - Hemorio
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
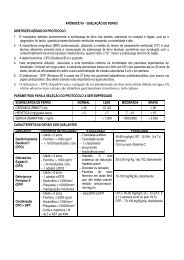
DDAVP INDICATIONS<br />
Desmopressin is more effective in patients with VWD type 1. In other subtypes, the response varies. In<br />
subtype 2A there is increase of FVIII, without, however, any change to TS. In subtype 2B and Platelet<br />
Type VWD or pseudo-von Willebrand Disease, Desmopressin is counter-indicated due to risk of transient<br />
plateletopenia occurrence. In subtype 2M, the response pattern is variable and the decision to apply<br />
Desmopressin will depend on the type of response to the test infusion. Desmopressin in subtype 2N<br />
results in high concentrations of FVIII, although it has a short half-life. Type 3 patients, in general, do not<br />
respond to Desmopressin.<br />
It is the chosen treatment for bleedings such as: epistaxis, hematuria, menorrhage, minor traumas and<br />
minor surgeries (dental extraction) in mild hemophilia patients and von Willebrand Disease Type 1 and 2A<br />
carries, the ones responding to DDAVP.<br />
ADMINISTRATION<br />
Desmopressin may be administered through subcutaneous, intravenous or intranasal routes. SUS<br />
(Brazilian Public Health System) makes DDAVP available, in IV presentation. The recommended dose for<br />
intravenous use, in slow infusion of 30 minutes, is 0.3 µg/kg, diluted in 50-100 ml saline solution.<br />
Maximum dose of 20 µg. The concentration peak of FVIII occurs after 30 to 60 minutes of the end of<br />
infusion. It may be repeated in 12 to 24 h. Subsequent doses present less effective responses due to the<br />
tachyphylaxis, because the pre-existing stock would be empty. However, there are studies which show<br />
that the response to the second dose is approximately 30% smaller than the one obtained from the first<br />
dose and in which there are no subsequent reductions in the next doses. The amount of doses applied<br />
should not exceed three.<br />
The recommended dose for subcutaneous use is the same (0.3 µg/kg), however applying to<br />
Desmopressin high concentration presentation (15-20 mcg/ampoule). For intranasal application the<br />
recommended dose is 300µg for adults and 150 µg for children. The use of subcutaneous and intranasal<br />
routes are convenient for the treatment of mild to moderate hemorrhages at home, although they are not<br />
yet made available by the Ministry of Health. After 30 to 60 minutes of Desmopressin administration<br />
intravenous, subcutaneous or intranasal) FVIII and FVW plasma concentrations increased from 3 to 5<br />
times in relation to baseline values. Overall, the response pattern to Desmopressin test is similar in a<br />
family, which can be a guidance to the type of response other family members will present, with no need<br />
to submit them to the therapeutic test.<br />
COLLATERAL EFFECTS<br />
Overall, the collateral effects have little relevance and are related to drugs vasomotor effects such as:<br />
facial redness, mild to moderate headache, hypotension/hypertension and tachycardia. Water retention<br />
and hyponatremia may also arise, due to antidiuretic effects of DDAVP.<br />
NOTES: Special attention must be given to:<br />
1. Elderly patients, due to cases of congestive cardiac failure;<br />
2. Children younger than 3-years-old, especially if receiving endovenous hypotonic solutions, due to the<br />
possibility of developing hyponatremia and convulsions;<br />
3. Patients experiencing unstable angina, due to reports of thromboembolitic phenomena;<br />
4. Carriers of VWD subtype IIB, they may present plateletopenia;<br />
5. Pregnant women, due to possibility of hypervolemia.<br />
COUNTER-INDICATION<br />
1. Patients with previous history of convulsion;<br />
2. Patients with arterial hypertension and cardiopathy;<br />
3. Patients Who developed plateletopenia after “test dose”;<br />
4. Patients with polydypsia.<br />
75