VOLUM OMAGIAL - Facultatea de Ştiinţe ale Naturii şi Ştiinţe Agricole
VOLUM OMAGIAL - Facultatea de Ştiinţe ale Naturii şi Ştiinţe Agricole
VOLUM OMAGIAL - Facultatea de Ştiinţe ale Naturii şi Ştiinţe Agricole
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Dinitrophenyl <strong>de</strong>rivates action on wheat germination / Ovidius University Annals, Biology-Ecology Series 14: 73-77 (2010)<br />
Number of<br />
plantlets<br />
Fig. 6. The height of the plantlets of the lots treated<br />
with L-β-phenylalanine<br />
Of all the five figures can be seen as only for<br />
seedlings lengths blank if the three lots are very close.<br />
In other cases the range of lengths of seedlings was<br />
higher. These differences show once again disrupting<br />
the <strong>de</strong>velopment of seedlings in the presence of<br />
chemicals, whether stimulatory or inhibitory effect.<br />
Toxicity<br />
16<br />
14<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
L-β-phenylalanine<br />
2.4-Dinitrophenol<br />
Lot A<br />
Lot B<br />
Lot C<br />
Average<br />
40 60 80 100<br />
Plantlets size (mm)<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0<br />
0 2 4 6 8 10<br />
Concentration (mM)<br />
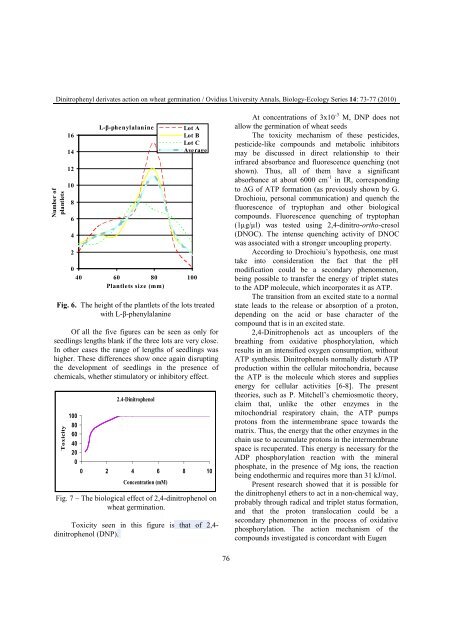
Fig. 7 – The biological effect of 2,4-dinitrophenol on<br />
wheat germination.<br />
Toxicity seen in this figure is that of 2,4dinitrophenol<br />
(DNP).<br />
76<br />
At concentrations of 3x10 -3 M, DNP does not<br />
allow the germination of wheat seeds<br />
The toxicity mechanism of these pestici<strong>de</strong>s,<br />
pestici<strong>de</strong>-like compounds and metabolic inhibitors<br />
may be discussed in direct relationship to their<br />
infrared absorbance and fluorescence quenching (not<br />
shown). Thus, all of them have a significant<br />
absorbance at about 6000 cm -1 in IR, corresponding<br />
to ∆G of ATP formation (as previously shown by G.<br />
Drochioiu, personal communication) and quench the<br />
fluorescence of tryptophan and other biological<br />
compounds. Fluorescence quenching of tryptophan<br />
(1µg/µl) was tested using 2,4-dinitro-ortho-cresol<br />
(DNOC). The intense quenching activity of DNOC<br />
was associated with a stronger uncoupling property.<br />
According to Drochioiu’s hypothesis, one must<br />
take into consi<strong>de</strong>ration the fact that the pH<br />
modification could be a secondary phenomenon,<br />
being possible to transfer the energy of triplet states<br />
to the ADP molecule, which incorporates it as ATP.<br />
The transition from an excited state to a normal<br />
state leads to the release or absorption of a proton,<br />
<strong>de</strong>pending on the acid or base character of the<br />
compound that is in an excited state.<br />
2,4-Dinitrophenols act as uncouplers of the<br />
breathing from oxidative phosphorylation, which<br />
results in an intensified oxygen consumption, without<br />
ATP synthesis. Dinitrophenols normally disturb ATP<br />
production within the cellular mitochondria, because<br />
the ATP is the molecule which stores and supplies<br />
energy for cellular activities [6-8]. The present<br />
theories, such as P. Mitchell’s chemiosmotic theory,<br />
claim that, unlike the other enzymes in the<br />
mitochondrial respiratory chain, the ATP pumps<br />
protons from the intermembrane space towards the<br />
matrix. Thus, the energy that the other enzymes in the<br />
chain use to accumulate protons in the intermembrane<br />
space is recuperated. This energy is necessary for the<br />
ADP phosphorylation reaction with the mineral<br />
phosphate, in the presence of Mg ions, the reaction<br />
being endothermic and requires more than 31 kJ/mol.<br />
Present research showed that it is possible for<br />
the dinitrophenyl ethers to act in a non-chemical way,<br />
probably through radical and triplet status formation,<br />
and that the proton translocation could be a<br />
secondary phenomenon in the process of oxidative<br />
phosphorylation. The action mechanism of the<br />
compounds investigated is concordant with Eugen