manual on management of drugs
manual on management of drugs
manual on management of drugs
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Unsatisfactory clinical resp<strong>on</strong>se, adverse reacti<strong>on</strong>s and bad storage<br />
c<strong>on</strong>diti<strong>on</strong>s:<br />
In respect <strong>of</strong> all problems <strong>of</strong> vaccines and sera relating to unsatisfactory<br />
clinical resp<strong>on</strong>ses, adverse reacti<strong>on</strong>s and exposure to bad storage c<strong>on</strong>diti<strong>on</strong>s has<br />
to be reported within 24 hours by teleph<strong>on</strong>e and followed up with samples as<br />
specified below with detail report relevant to the product (lot number, expiry date,<br />
manufacture etc.), storage c<strong>on</strong>diti<strong>on</strong>s, maintenance <strong>of</strong> the cold chain, reacti<strong>on</strong>s<br />
caused, dosage applied etc (sample sending form appears in annexure XVI can be<br />
used) addressed to the Nati<strong>on</strong>al C<strong>on</strong>trol Laboratory (Head / Dept <strong>of</strong> Vaccine<br />
Quality C<strong>on</strong>trol, MRL C<strong>on</strong>tact No. 2698660, 2693532-4 Res. 2597723). Advice<br />
and approval regarding further use <strong>of</strong> the batches should be sought from the<br />
Epidemiologist for all EPI vaccines and from the Director Medical Supplies<br />
Divisi<strong>on</strong> for all N<strong>on</strong>-EPI vaccines.<br />
Please note that the NCL should be c<strong>on</strong>tacted prior to sending the samples.<br />
Sample size for Q.A test-<br />
(1) Single dose vials: sterility test - 5 vials, safety test - 20 vials (2) Multiple dose<br />
vials:<br />
sterility test - 3 vials, safety test - 3 vials<br />
N.B. To be sent in a vaccine carrier maintaining cold chain with copies <strong>of</strong> all<br />
relevant documents.<br />
(2) Same procedure should be followed as pharmaceuticals regarding the handling<br />
<strong>of</strong> product recalling.<br />
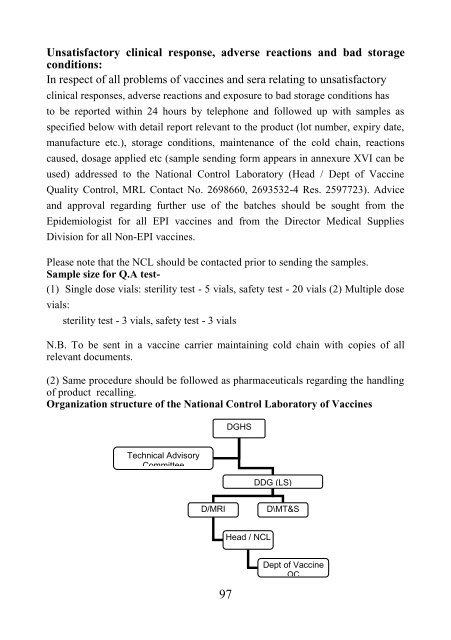
Organizati<strong>on</strong> structure <strong>of</strong> the Nati<strong>on</strong>al C<strong>on</strong>trol Laboratory <strong>of</strong> Vaccines<br />
DGHS<br />
Technical Advisory<br />
Committee<br />
DDG (LS)<br />
D/MRI<br />
D\MT&S<br />
Head / NCL<br />
97<br />
Dept <strong>of</strong> Vaccine<br />
QC