SOFT 2004 Meeting Abstracts - Society of Forensic Toxicologists
SOFT 2004 Meeting Abstracts - Society of Forensic Toxicologists
SOFT 2004 Meeting Abstracts - Society of Forensic Toxicologists
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
F20 <br />
CONFIRMATION RATES OF INITIAL DRUG ASSAYS IN A GROUP OF HHS-CERTIFIED<br />
LABORATORIES, JANUARY 01 THROUGH DECEMBER 31, 2003<br />
II: NON-REGULATED SPECIMENS<br />
Craig A. Sutheimer l *, Michael R. Baylor I, John Irvingl, John M. Mitchell\ Donna M. Bush 2 : IRTI<br />
International, Research Triangle Park, NC, USA; 2DWP, SAMHSA, Rockville, MD, USA<br />
The specificity <strong>of</strong> immunoassays associated with urine drug testing has long been a subject <strong>of</strong> discussion among<br />
forensic toxicologists. While it has been known that some drug class immunoassays have very high rates for the<br />
confirmation <strong>of</strong> presumptive positives, it is also recognized that other drug class immunoassays produce a<br />
significant number <strong>of</strong> presumptive positives that fail to confirm when subjected to confirmatory testing by<br />
GCJMS. These observations are further confounded as initial and confirmatory drug test cut<strong>of</strong>f concentrations<br />
change and as the number <strong>of</strong> drug analytes in confirmatory panels are broadened. These observations led to an<br />
examination <strong>of</strong> the immunoassays currently in use at drug testing laboratories. The goal <strong>of</strong> this study was to<br />
document the possible differences in specificities and cross-reactivities <strong>of</strong> the technologies with multiple cut<strong>of</strong>fs<br />
in both the initial and confirmation testing procedures in addition to possible variability in the analytes defined in<br />
the confirmatory panels.<br />
The study included data from 10 laboratories certified by the U. S. Department <strong>of</strong> Health and Human Services<br />
(HHS) encompassing over 10 million specimens tested during 2003. These specimens were not subject to the<br />
criteria <strong>of</strong> the Mandatory Guidelines for Federal Workplace Drug Testing Programs. The data was obtained from<br />
laboratories that used CEDI A, EIA and KIMS technologies as a primary initial test, with varying initial and<br />
confirmatory test cut<strong>of</strong>fs. Some laboratories conducted additional screening <strong>of</strong> presumptive positives with a<br />
second initial test. Summaries <strong>of</strong> specimen testing and confirmation rates are presented in the tables below. The<br />
confirmation rates are expressed as percent <strong>of</strong> the presumptive positives confirmed by GC/MS for each drug<br />
class. The lowest and highest confirmation rate for each "initial/confirmatory testing cut<strong>of</strong>f pair" identified is<br />
provided.<br />
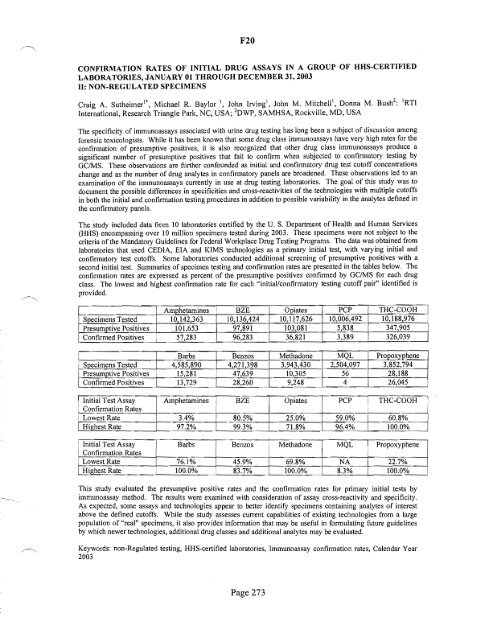
Amohetamines BZE<br />
Specimens Tested 10 142,363 10,136424<br />
Presumptive Positives 101,653 97,891<br />
Confirmed Positives I 57,283<br />
96,283<br />
Opiates PCP<br />
10,117,626 10 006,492<br />
103,081 5,838<br />
36,821 3,389<br />
I<br />
THC-COOH<br />
10,188,976 i<br />
347,905 I<br />
326,039 1<br />
T Barbs I Benzos Methadone MQL Propoxyphene<br />
. Specimens Tested T 4,585,890 I 4,271,398 3,943430 2,504,097 3,852,794<br />
Presumptive Positives I 15,281 47,639 10,305 56 28,188<br />
Confirmed Positives T 13,729 L 28,260 9,248 4 26,045<br />
I Initial Test Assay<br />
: Confirmation Rates<br />
Amphetamines BZE Opiates PCP I THC-COOH<br />
i Lowest Rate 3.4% 80.5% 25.0% 59.0% I 60.8%<br />
I Highest Rate 97.2% 99.3% 71.8% 96.4% l 100.0%<br />
I<br />
Initial Test Assay Barbs Benzos Methadone MQL ! Propoxyphene<br />
I Confirmation Rates<br />
-<br />
i Lowest Rate 76.1% 45.9% 69.8% NA 22.7%<br />
I<br />
I Highest Rate i 100.0% 83.7% 100.0% 8.3% 100.0~<br />
This study evaluated the presumptive positive rates and the confirmation rates for primary initial tests by<br />
immunoassay method. The results w~re examined with consideration <strong>of</strong> assay cross-reactivity and specificity.<br />
As expected, some assays and technologies appear to better identifY specimens containing analytes <strong>of</strong> interest<br />
above the defined cut<strong>of</strong>fs. While the study assesses current capabilities <strong>of</strong> existing technologies from a large<br />
population <strong>of</strong> "real" specimens, it also provides information that may be useful in formulating future guidelines<br />
by which newer technologies, additional drug classes and additional analytes may be evaluated.<br />
Keywords: non-Regulated testing, HHS-certified laboratories, Immunoassay confirmation rates, Calendar Year<br />
2003<br />
Page 273