SOFT 2004 Meeting Abstracts - Society of Forensic Toxicologists
SOFT 2004 Meeting Abstracts - Society of Forensic Toxicologists
SOFT 2004 Meeting Abstracts - Society of Forensic Toxicologists
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
P42 <br />
ENANTIOMERIC ANALYSIS OF EPHEDRINES AND NOREPHEDRINES<br />
2<br />
Sheng-Meng Wang l • 2 , Russell J. Lewis' 2 , Dennis Canfield 2 , and Ray H. LiU .J. 4 , 'Central Police University,<br />
Taoyuan, Taiwan. 2Toxicology and Accident Research Laboratory, FAA Civil Aerospace Medical Institute,<br />
Oklahoma City, OK, U.S.A. JDepartment <strong>of</strong> medical technology, Fooyin University, Kaohsiung Hsieh,<br />
Taiwan. 4Department <strong>of</strong>Justice Sciences, University <strong>of</strong>Alabama at Birmingham, Birmingham, AL, U.S.A.<br />
Concerned with variations in abuse potential and control status among various isomers <strong>of</strong> ephedrines and<br />
norephedrines, this study was conducted to develop an effective method for the simultaneous analysis <strong>of</strong><br />
nine structurally related ephedrine-type compounds. Select cold medications were then analyzed to<br />
characterize the enantiomeric compositions <strong>of</strong> ephedrine, norephedrine (phenylpropanolamine or PPA),<br />
pseudoephedrine, norpseudoephedrine (cathine), and cathinone.<br />
Among various approaches investigated, a 60-m HP-5MS (0.25 mm!D, 0.25 !!m film thickness) was found<br />
to successfully resolve the following compounds <strong>of</strong> interest that were derivatized with (-)-a-methoxy-a.<br />
trifloromethy lpheny lacetic acid (MTPA): (+ )-cathine, (+ )-cathinone, (-)-cathinone, (+)-ephedrine, (-)<br />
ephedrine, (+)-PPA, (-)-PPA, (+)-pseudoephedrine, (-}-pseudoephedrine. A (-}-cathine standard was not<br />
available, but should also be resolvable using this analytical procedure. The injector temperature was set at<br />
250°C and the oven pr<strong>of</strong>ile was 160-220°C at 5°C/min, hold 1 min, 220-250°C at 25°C/min. This method<br />
was applied to the analysis <strong>of</strong> various over-the-counter cold medications and the results derived from three<br />
samples are shown in Table 1. These compounds were isolated from the cold medications utilizing a<br />
simple liquid/liquid extraction with ethyl acetate. This method has proven to be an efficient procedure for<br />
the separation and identification <strong>of</strong>various enantiomeric ephedrine and norephedrine-type compounds.<br />
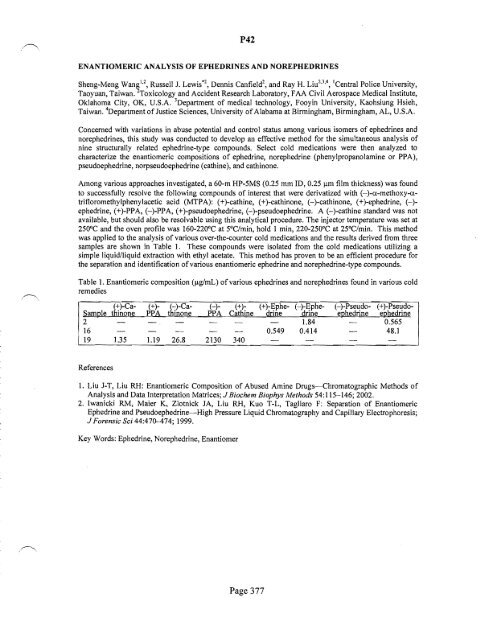
Table 1. Enantiomeric composition (JlglmL) <strong>of</strong> various ephedrines and norephedrines found in various cold<br />
remedies<br />
(+)-Cathinone<br />
PPA thinone PPA Cathine (+)-Ephedrine<br />
(+) H-Ca<br />
(-) (+)-<br />
SamJ;!le<br />
H-Ephedrine<br />
(-)-PseudoeJ;!hedrine<br />
(+)-PseudoeJ;!hedrine<br />
2 1.84 0.565<br />
16 0.549 0.414 48.1<br />
19 1.35 1.19 26.8 2130 340<br />
References<br />
1. Liu J-T, Liu RH: Enantiomeric Composition <strong>of</strong> Abused Amine Drugs--Chromatographic Methods <strong>of</strong><br />
Analysis and Data Interpretation Matrices; J Biochem Biophys Methods 54:115-146; 2002.<br />
2. Iwanicki RM, Maier K, Zlotnick JA, Liu RH, Kuo T-L, Tagliaro F: Separation <strong>of</strong> Enantiomeric<br />
Ephedrine and Pseudoephedrine-High Pressure Liquid Chromatography and Capillary Electrophoresis;<br />
J <strong>Forensic</strong> Sci 44:470--474; 1999.<br />
Key Words: Ephedrine, Norephedrine, Enantiomer<br />
Page 377