ECOPROBE 5 - rs dynamics
ECOPROBE 5 - rs dynamics
ECOPROBE 5 - rs dynamics
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
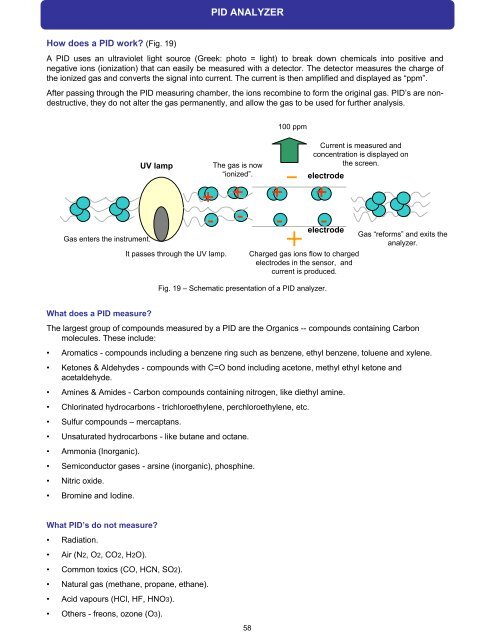
How does a PID work? (Fig. 19)<br />
A PID uses an ultraviolet light source (Greek: photo = light) to break down chemicals into positive and<br />
negative ions (ionization) that can easily be measured with a detector. The detector measures the charge of<br />
the ionized gas and converts the signal into current. The current is then amplified and displayed as “ppm”.<br />
After passing through the PID measuring chamber, the ions recombine to form the original gas. PID’s are nondestructive,<br />
they do not alter the gas permanently, and allow the gas to be used for further analysis.<br />
Gas ente<strong>rs</strong> the instrument.<br />
UV lamp<br />
It passes through the UV lamp.<br />
The gas is now<br />
“ionized”.<br />
58<br />
Current is measured and<br />
concentration is displayed on<br />
the screen.<br />
electrode<br />
electrode<br />
Gas “reforms” and exits the<br />
analyzer.<br />
Charged gas ions flow to charged<br />
electrodes in the sensor, and<br />
current is produced.<br />
What does a PID measure?<br />
The largest group of compounds measured by a PID are the Organics -- compounds containing Carbon<br />
molecules. These include:<br />
• Aromatics - compounds including a benzene ring such as benzene, ethyl benzene, toluene and xylene.<br />
• Ketones & Aldehydes - compounds with C=O bond including acetone, methyl ethyl ketone and<br />
acetaldehyde.<br />
• Amines & Amides - Carbon compounds containing nitrogen, like diethyl amine.<br />
• Chlorinated hydrocarbons - trichloroethylene, perchloroethylene, etc.<br />
• Sulfur compounds – mercaptans.<br />
• Unsaturated hydrocarbons - like butane and octane.<br />
• Ammonia (Inorganic).<br />
• Semiconductor gases - a<strong>rs</strong>ine (inorganic), phosphine.<br />
• Nitric oxide.<br />
• Bromine and Iodine.<br />
What PID’s do not measure?<br />
• Radiation.<br />
• Air (N2, O2, CO2, H2O).<br />
• Common toxics (CO, HCN, SO2).<br />
• Natural gas (methane, propane, ethane).<br />
• Acid vapou<strong>rs</strong> (HCl, HF, HNO3).<br />
• Othe<strong>rs</strong> - freons, ozone (O3).<br />
PID ANALYZER<br />
100 ppm<br />
Fig. 19 – Schematic presentation of a PID analyzer.